Orgaanisen kemian laudaturtyöt
advertisement

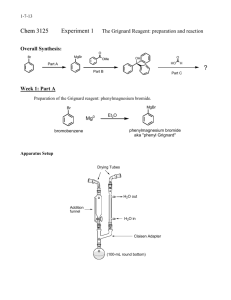
Orgaanisen kemian laudaturtyöt 3.paketti Orgaanisen kemian laboratorio Kemian laitos Helsingin yliopisto 2005 Laudaturtöiden synteesit ja analyysi Laudaturtöissä tehdään itsenäisesti viisi synteesiä sekä kvalitatiivinen analyysi. Analyysi sisältää vähintään 3 tuntematonta ainetta, jotka opiskelijan tulee erottaa toisistaan ja tunnistaa fysikaalisten vakioiden ja spektroskooppisten menetelmien perusteella. Analyysistä laaditaan erillinen selostus ja se kuuluu kaikkiin työpaketteihin. Jokaiseen synteesipakettiin kuuluu sekä suojakaasun käyttöä vaativa työ että tuotteen flash-kromatografinen puhdistus. Lue etukäteen näihin liittyvät monisteet. LAH reduction of p-anisaldehyde.................................................................................................. 5 4,4-Diphenyl-3-buten-2-one.......................................................................................................... 6 4-Methoxymethylbenzoic acid ...................................................................................................... 9 Phenetole .................................................................................................................................... 11 Diphenylacetaldehyde ................................................................................................................. 13 Analysis ...................................................................................................................................... 14 2 LAH reduction of p-anisaldehyde Reagents 0.8 g of of LiAlH4 (one pellet) 1 mL of anisaldehyde Safety Notes Equipment reflux apparatus with LiAlH4 is flammable, handle it only two-necked flask and a in dry conditions. Don't grind the septum pellet under air! ballons Diethyl ether is flammable and an three-way stopcock irritant. syringes + needles ice-water bath Procedure All apparatus must be thoroughly dried in a hot oven (>120°C) before use. Flush a 50 mL twoneck round-bottomed flask (a magnetic stirrer bar inside) with a stream of argon for about 2 minutes. Close one neck with a septum and the other with a stopper. LiAlH4 is weighed directly inside the flask, and the flask is kept closed. The flask is equipped with a reflux condenser, and an argon balloon is put in top of the condenser by a three-way stopcock. A vacuum is sucked into the flask via a t hree-way stopcock and the flask is filled with argon from the balloon. Repeat this operation three times and leave the stopcock open to the balloon. Add the dry ether (25 mL) through the septum with a syringe needle over LiAlH4 at such a rate that the mixture refluxes gently. Stir for five minutes and add anis aldehyde dropwise through a syringe needle (caution: vigorous reaction). Allow the mixture to stir at roo m temperature for about one hour after the addition. The reaction must be controlled with TLC (small sample from reaction mixture is put in the test-tube containing water. Add ether, s hake and take TLC-sample from the organic phase). A ttention! If you have used LiAlH4-pellet, make sure it is completely reacted before the next step. Isolation and purification Cool the flask in an ice-water bath for 10 m inutes, and add ab out 1 mL of water through the septum (caution!). Stir the mixture for about 5 minutes, and add ab out 6 mL of 2 M s odium hydroxide in the same way. Stir for about 5 minutes. Add 25 mL of ether, shake gently and decant the ether from the residual inorganic salts. Repeat with further 25 mL of ether. Combine the ether phases and dry with Na2SO4. Remove the solvent on the rotary evaorator. Yield is 80%. Product characterization Run an IR spectrum of the product. 5 4,4-Diphenyl-3-buten-2-one Grignard and Organolithium Reagents Although organometallic reagents involving many different metals have found application in organic synthesis, those based on magnesium and lithium have probably found the widest use. Organomagnesium reagents, more commonly known as Grignard reagents after their discoverer the Frenchman Victor Grignard, who received the Nobel prize in 1912, are particularly suited for use in the o rganic laboratory since they are easily prepared by reaction of an alkyl or aryl halide with magnesium metal in an ether solvent. Organolithium compounds on the other hand are more difficult to handle, requiring the use o f rigorously anhydrous solvents under an inert atmosphere. They are made by the react ion of halides with lithium metal, although many organolithium reagents are co mmercially available as stock solutions in inert so lvents. Whilst, for practical purposes the structures of organometallic compounds can be regarded as monomeric, in fact the structures are much more complicated, and involve aggregates. Grignard Reagents: Addition of Phenyl Magnesium Bromide to Ethyl Acetoacetate Ethylene Acetal Esters react readily with an excess of a Grignard reagent to give tertiary alcohols. This experiment illustrates the addition of phenylmagnesium bromide, prepared f rom bromobenzene and magnesium turnings, to the ethylene acetal of ethyl acetoacetate. It is essential for the reactive ketone group of eth yl acetoacetate to be protected from reaction with the Gr ignard reagent. In a second, optional step the ketal protecting group is removed by acid hydrolysis, to give the tertiary keto alcohol which spontaneously dehydrates under the acidic conditions to the -unsaturated ketone 4,4-diphenylbut-3-en-2-one. The final product can be purified by column chromatography if desired. Further Reading: Morrison & Bo yd p. 883 ; Streitwieser & Heathcock p. 497; Carey & Sundberg p. B259; McMurray p. 594; Fessenden & Fessenden p. 652; March p. 825; Vollhardt p. 807; Wade pp. 424, 1071. Reference: This procedure is based on D.R. Paulson, A.L. Hartwig. 6 Reagents 1. Grignard reagent 0.45 g of magnesium turnings dry diethyl ether 1-2 crystals iodine 2.62 g of bromobenzene 1.45 g of ethyl acetoacetate ketal light petroleum 2. Hydrolysis conc. hydrochloric acid acetone diethyl ether sodium bicarbonate solution (saturated) silica gel Safety Notes flammable flammable, irritant corrosive, lachrymator irritant irritant flammable flammable Equipment reflux apparatus with addition funnel separation/extraction suction filtration apparatus apparatus for column cromatography corrosive flammable flammable, irritant irritant dust Procedure 1. Preparation and reaction of the Grignard reagent All apparatus must be thoroughly dried in a h ot (>120 ° C) oven bef ore use, for example over night. Set up a 100 m L round bottomed flask with a dropping funnel, a stirring bar and a reflux condenser equipped with a calcium chloride tube. Then add the magnesium turnings, 10 mL of dry ether and a chrystal of iodine to the flask. Place 10 mL of dry ether and the bromobenzene in the addition funnel, and add a few drops of this solution to the magnesium. Start the stirrer, and wait until the formation of the Grignard reagent starts. The start of the reaction will be apparent; the ether starts to reflux, and takes on a gray-brown appearance. Add the remaining bromobenzene solution, diluted with an ext ra 20 mL of ether, at such a rate as t o maintain a gentle reflux. After the addition is complete, reflux the mixture with stirring on a hot water bath for about 10 min. Cool the flask in an i ce bath, and then add a solution of the ethyl acetoacetate ketal in 10 mL of dry ether dropwise. After the addition is complete, stir the mixture for a further 30 min at room temperature. After a wh ile, the product precipitates causing the magnetic stirrrer to jam; let the mixture rest for 30 min. Then add 20 mL of ice-water to the flask. When the ice has melted, add a further 10 mL of ether, and stir the mixture until the gummy solid dissolves. Some extra ether may be needed, also add some dilute HCl until the solution is acidic. Cool the flask during the addition of acid. Transfer the mixture to a separatory funnel, and separate the layers. Extract the aqueous layer with 10 mL of ether, combine the ether layers, wash them with 10 mL of water and dry over MgSO4 . Filter off the drying agent and evaporate the filtrate on the rotary evaporator to leave a yellow-orange oil. Record the yield, mp, IR and NMR (CDCl3) 7 2. Hydrolysis of the ketal: 4,4-diphenylbut-3-en-2-one Place the above tertiary alcohol, 1 mL of conc. HCl, 25 mL of acetone and 1.5 mL of water in a 50 mL round bottomed flask. Fit a condenser, and heat the mixture under reflux for 1 hour. Transfer the cooled mixture to a separatory funnel, add 25 mL of water, and extract it with 2x15 mL of ether. Combine the ether layers, wash them with 15 mL of saturated sodium bicarbonate solution (Care! CO2 gas evolved), 15 mL of water, and dry them over MgSO4 . Filter off the drying agent, and evaporate the filtrate on the rotary evaporator to leave the crude product. Isolation and purification Purify the product by column chromatography on silica gel using 10:1 hexane:EtOAc as eluent. Remove the eluent and dry the product at the oil pump. Product characterization Record the yield, and the IR and NMR (CDCl3). The yield should be 20 %. Questions 1. Write the reaction mechanism for the acid hydrolysis of the acetal protecting group, and the subsequent dehydration of the resulting keto alcohol. 2. Assign the spectroscopic data for your product(s). 3. Suggest an alternative synthesis of 4,4-diphenylbut-3-en-2-one. 8 4-Methoxymethylbenzoic acid A convenient procedure for generating bromine radicals utilizes N-bromosuccinimide as t he bromine source and benzoyl peroxide as the initiator. Unlike most benzylhalides, the 4-bromomethylbenzoic acid prepared in this experiment is not lachrymatory, making it suitable as a laboratory procedure. Nonetheless, the product is a potential skin irritant and due caution should be exercized in its handling. Reagents 1. Preparation of 4-bromomethylbenzoic acid Safety Notes Equipment stirrer/hotplate reflux apparatus - toxic - irritant - an oxidizing agent and liable to explode if heated or ground as the dry solid. Handle with extreme caution! chlorobenzene - flammable light petroleum (bp. 40-60 C°) - flammable 2.72 g of 4-methylbenzoic acid 3.60 g of N-bromosuccinimide 0.20 g of benzoyl peroxide 2. Preparation of 4-methoxymethylbenzoic acid methanol potassium hydroxide pellets - flammable, toxic - corrosive Preparation of 4-bromomethylbenzoic acid Procedure: place the 4-methylbenzoic acid and N-bromosuccinimide in a 100 mL round bottomed flask. Add the benzoyl peroxide, taking care that none sticks to the ground glass joint of the flask. Finally, add 25 m L of chlorobenzene by pipet and wash down any solids which may be adhering to the sides of the flask, paying particular attention to the ground glass joint. Arrange the apparatus for reflux and heat gently for 1 h wi th occasional (ca. every 5 min) vigorous swirling in order to ensure good mixing. Isolation and purification: cool the flask and contents in an ice bath (ca. 10 min) and filter off the precipitated products with suction. Wash the residue on the f unnel with light petroleum (3 x 10 mL), and transfer the solid to a beaker. Add water (50 mL), stir the the slurry thoroughly to dissolve the succinimide present, and filter the mixture under suction, washing the s olid residue successively with water (2 x 10 m L) and light petro leum (2 x 10 mL). Leave the product on the 9 funnel with suction to dry as thoroughly as possible and reco rd the yield and melting point of the crude material. The crude mater ial is of sufficient purity to use in the following experiment but, if time permits, may be recrystallized from ethyl acetate. Product charaterization: measure the melting point and run IR and NMR spectra of your product. Preparation of 4-methoxymethylbenzoic acid Procedure: place the potassium hydroxide pellets and the methanol in a 100 mL round bottomed flask and the 4-bromomethylbenzoic acid prepared in the previous experiment. Set the apparatus for reflux with a calcium chloride guard tube and heat gently for 45 min. Remove the methanol at reduced pressure on the rotary evaporator with gentle warming and dissolve the alkaline residue in water (30 mL). Isolation and purification: acidify the mixture with dilute hydrochloric acid and filter the precipitated product. Wash the solid with light petroleum (2 x 15 mL) and and dry with suction on the funnel. Record the yield and melting point of your crude product and then recrystallize it from water. As before, filter the precipitated product with suction, wash the crystalline solid with light petroleum (2 x 15 mL) and dry on the funnel with suction. Compare the purified product's melting point with that of the crude material. The yield is 46% (calculated from 4-methylbenzoic acid). The melting point is 123°C. Product characterization: measure the melting point and run IR and NMR spectra of the purified product 10 Phenetole The objective of the experiment is to prepare phenetole by Williamson synthesis from phenol using a phase-transfer catalyst. The work of Makosza, Starks, and other investigators has led to t he use of phase-transfer catalysts to accelerate reactions in two-phase systems. For example, the displacement of halide ions by nucleophiles such as cyanide can be catalyzed by quat ernary ammonium ions, which transport cyanide ion from the aqueous to the organic phase where it reacts with the alkyl halide. If the method works for cyanide ion, it should work for other nucleophiles as we ll. The old and familiar Williamson synthesis of ethers involves the displacement of halide ion (and other leaving groups) by nucleophiles such as alkoxide and aryloxide ions. It is most successful in combining a primary halide with a sodium alkoxide or aryloxide. Sodium alkoxides are usually prepared by adding bits of sodium metal to the corresponding alcohol. Then the alkyl halide is added and the mixture is refluxed for five hours or so to make the ether. Sodium aryloxides can be prepared by treating the corresponding phenol (which is much more acidic than an alcohol) with the less hazardous base, sodium hydroxide. Unfortunately, alkyl halides will not dissolve in an aqueous solution of NaOH, so the aryloxides are more often prepared by adding sodium to the phenol in an organic solvent such as ethano l or benzene, followed by the addition of the alkyl halide. When the phase-t ransfer-catalysis technique is used, however, sodium phenoxide is prepared in the aqueous phase by mixing the phenol with sodium hydroxide, while the alkyl h alide is dissolved in an organic solvent such a s methylene chloride. When the catalyst is added and the two-phase system is s tirred, the phenoxide is carried into the organic phase by the quaternary ammonium cation (Q+), where it can react with the halide to form a phenyl ether. Return of Q+ to the aqueous phase to pick up more ArO- completes the cycle. Literature: Lehman, J. W. Operational Organic Chemistry, p. 270 Reagents 25 mmol phenol 50 mmol ethyl bromide 1 g of tetrabutyl ammonium hydrogensulphate Safety Notes Equipment reflux apparatus Phenol is toxic and corrosive and can separation/extraction severely burn the skin and eyes; ingestion distillation apparatus of as little as one gram has proven fatal. Avoid any contact with skin, eyes, and clothing. Ethyl bromide is toxic by ingestion, inhalation, and skin absorption; it is also flammable and the vapor is irritaing to eyes and lungs. Avoid contact with the liquid, do not inhale vapors, and keep away from heat or flames. Methylene chloride vapors may be harmful and the liquid is dangerous to the eyes; avoid prolonged contact with the liquid and inhalation of vapors. 11 Procedure Calculate the mass of crystalline phenol or 90% aqueous phenol needed to provide 25 mmol of phenol, and the volume of 50 mmol of ethyl bromide. In a 100 mL round-bottomed flask, dissolve 25 mmol of phenol in 25 mL of 2 M aqueous sodium hydroxide and add 25 mL o f methylene chloride, 50 mmol of ethyl bromide (volatile!), and 1 g of of tetrabutylammonium hydrogensulphate. Reflux the mixture gently (in a water bat h) for about four hours with vigorous mechanical stirring, using two reflux condensers in series. Follow the process of the reaction by TLC in methyl chloride. Isolation and purification Separate and save t he organic (lower) layer, then extract the aqueous layer with two 20 mL portions of methylene chloride and combine the extracts with the reserved organic layer. Wash the organic solution with 25 mL o f 2 M aqueous sodium hydroxide, then with 25 mL of saturated aqueous sodium chloride. Dry the organic solution and evaporate the solvent using a solvent trap. Purify the crude phenetole by micro distillation (bp. 170°C). A dense white fog may form in the boiling flask near the end o f the distillation - heating should be stopped at this point. Weigh the product. The yield is 80%. Product characterization Run a GC of the product. 12 Diphenylacetaldehyde Literature: Danilov, S.; Venus-Danilova, E. Chemische Berichte 1926, 59B, 1032-43 (procedure on page 1041). Reagents Equipment 8.0 g of hydrobenzoin reflux apparatus 40.0 g of oxalic acid separation/extraction reduced pressure distillation apparatus Procedure 8 g of of hydrobenzoin (mp. 134°C) and 40 g of of crystalline oxalic acid are heated in an oil bath for 4 hours (oil bath temperature: 156°C). Isolation and purification The obtained product is extracted with ether, washed with sodium hydroxide solution and dried with sodium sulphate. It is first distilled under reduced pressure at a temperature of 184-186°C/20 mmHg. The dis tilled fluid is distilled anew (if necessary), at a temperature of 188-188.5°C/24 mmHg. The yield is 60%. Product characterization Run IR and NMR spectra of the product. 13 Analysis Analysis consists of at least 3 substances which are to be separated and purified by extraction and distillation/recrystallisation and identified by physical properties and spectroscopic methods. When analysis is carried out and all the components are identified, the student has to write a report, which includes analysis form (names, physical data, structures etc) written report describing separation and identification processes for each substance a drawing of separation scheme describing overall separation process melting point printouts IR- and NMR-spectra with interpretation The report is returned to the assistants, who check whether the analysis is correct and whether the report is accepted. If the analysis is not correct, assistant will help to find out the problematic or uncertain parts of the separation / identification process. 14