Reinhard Laubenbacher Virginia Bioinformatics Institute and
advertisement
Complex Models in Systems Biology
Program on Development, Assessment, and Utilization of
Complex Computer Models
SAMSI
September 11, 2006
Reinhard Laubenbacher
Virginia Bioinformatics Institute
and
Mathematics Department
Virginia Tech
The Hallmarks of Cancer Hanahan & Weinberg (2000)
Biochemical Networks
Metabolic space
Metabolite 1
Protein 2
Metabolite 2
Protein space
Complex 3:4
Protein 4
Protein 3
Protein 1
Gene 2
Gene 3
Gene 1
Gene space
Gene 4
Brazhnik, P., de la Fuente, A. and Mendes, P. Trends in Biotechnology 20, 2002
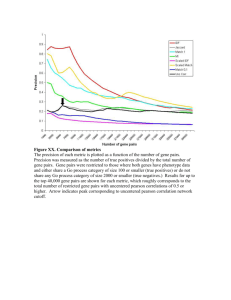
System-Level Experimental Data
Systems biology
• New technology allows system level
measurements in molecular biology.
• Can build system-level models.
• Need to scale up modeling technology.
The dynamic GNS simulation of interconnected signal
transduction pathways and gene expression networks
controlling human cell growth contains over 2,000
variables. The model describes the processes of
endocytosis, receptor signaling, signal transduction,
transcriptional control of gene expression networks,
and protein translation and degradation mechanisms.
It predicts various physiological outcomes such as
cell cycle progression and arrest through G1-S and
G2-M starting from mitogenic signaling, cell cycle
arrest and apoptosis induction via p53, and the
interplay between survival signals and apoptosis.
Gene Network Sciences
http://www.gnsbiotech.com/news-press020603.html
• fruitfly image
www.ars-grin.gov/mia/images/News/
Wildtype Gene Expression
Nature 406 2000
A Boolean network is a time-discrete dynamical
system
f=(f1, … ,fn): {0, 1}n → {0, 1}n.
Each fi is a Boolean function. Dynamics is
generated by iteration. For a binary vector x
we have
f(x) = (f1(x), …, fn(x)),
that is, the variables are updated synchronously.
A Mathematics Program
• Study stochastic sequential dynamical systems
of the form
f=(f1, … ,fn): kn → kn
where k is a finite field, and fi: kn → kn, which
only changes the ith coordinate. An update of
the system is computed by choosing an update
order of the variables based on a probability
distribution on update orders. That is,
f(x) = fi ◦ fj ◦ …
Fact: Each fi can be represented uniquely as a
polynomial function.
“Bottom-up modeling:” Model individual
pathways and aggregate to system-level
models
“Top-down modeling:” Develop network
inference methods for system-level
phenomenological models
Model Types
Ideker, Lauffenburger, Trends in Biotech 21, 2003
Challenges
Cellular biochemical networks are
• Nonlinear
• High-dimensional
• Poorly understood
• Underdetermined by available data, which are
typically noisy
• Difficult to perturb
Network inference
Problem: Given D={(si, ti) ∈ kn×kn }, find the
“most likely” model f: kn → kn such that
f(si) = ti
Using methods from computational algebra, one
can describe the entire space of possible
models in a compact way and choose a most
parsimonious model by optimizing model
structure.
R. Laubenbacher and B. Stigler, A
computational algebra approach to the reverseengineering of gene regulatory networks, J.
Theor. Biol. 229 (2004)
A. Jarrah, R. Laubenbacher, B. Stigler, and M.
Stillman, Reverse-engineering of polynomial
dynamical systems, Adv. in Appl. Math. (2006)
in press
Application
Use the Albert-Othmer Boolean model to
generate time courses (wild-type and knockout mutant) totaling 24 time courses of 7 data
points each. (Note that the system has 221
possible states.)
The reverse-engineering algorithm recovers the
“wiring diagram” of the network correctly, as
well as 19 of the 21 Boolean functions.
Summary
• Understanding cellular networks is very
important (e.g., personalized medicine).
• System-level data are increasingly available
and increasingly quantitative.
• Many mathematical and computational
problems are waiting to be solved.
• There is a large community of life scientists
eager to collaborate.