thermal.notebook 1 April 18, 2013
advertisement

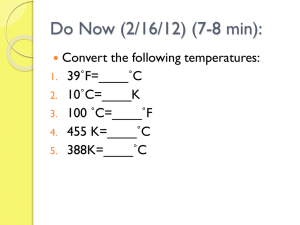
thermal.notebook April 18, 2013 Physics 11 Thermal Energy Worksheet Q=mc(Tf ­Ti) Name:_______________ Tf=(m1c1Ti1+m2c2Ti2+m3c3Ti3)/(m1c1+m2c2+m3c3) cwater=4180 csilver=235 clead=130 czinc=388 ciron=450 ccopper=385 (all in J/(kg oC) 1. How much energy is needed to heat 50 kg of lead from 8 to 52 degrees C ? 2. If 345 J of thermal energy is added to 0.345 kg of zinc at 8oC, how warm does it get? The rules for sig. figs. here: 345/(.345*388) = 2.57...is a 3 sig fig calculation, and when added to 8, the rule is align the decimal and add, then correct the trailing unsure digits BUT KEEP ALL the digits "in front" so 8.x + 2.57 = 10.57 but the 5 is uncertain, rounds to 11 degrees 3. If 1.50 kg of a liquid is heated from 31oC to 53oC when 12 000 J of thermal energy are added, what is the specific heat capacity of the liquid? 4. What is the final temperature if 0.20 kg of water(coffee) at 92 degrees C is cooled by a 21 g silver spoon at 20 degrees and 0.015 kg of 4 degree water? 5. How long does it take a 550 W heater to raise 8.5o water to its boiling point (100o)? 6. A tub contains 103 kg of hot water at 45o What mass of cold water at 11o is added to bring the final temperature of the mixture down to 39o ? Rather than taking the Tf = (m1c1Ti1 + m2c2Ti2...) formula and trying to re­arrange for m2, go back to how that formula was developed... heat lost by hot = heat gained by cold ∆Q = ­∆Q Then plug in the parts: 103 kg * 4180 J/kgoC * (39oC ­ 45oC) = ­ m * 4180 J/kgoC * (39oC ­ 11oC) Notice the 4180 can be cancelled from both sides, and the whole thing becomes: 103 * ­6 = ­ m * (28) or m = 22.07...kg 1