Photoelectron Spectroscopy Info - AP Chemistry Resources for the
advertisement
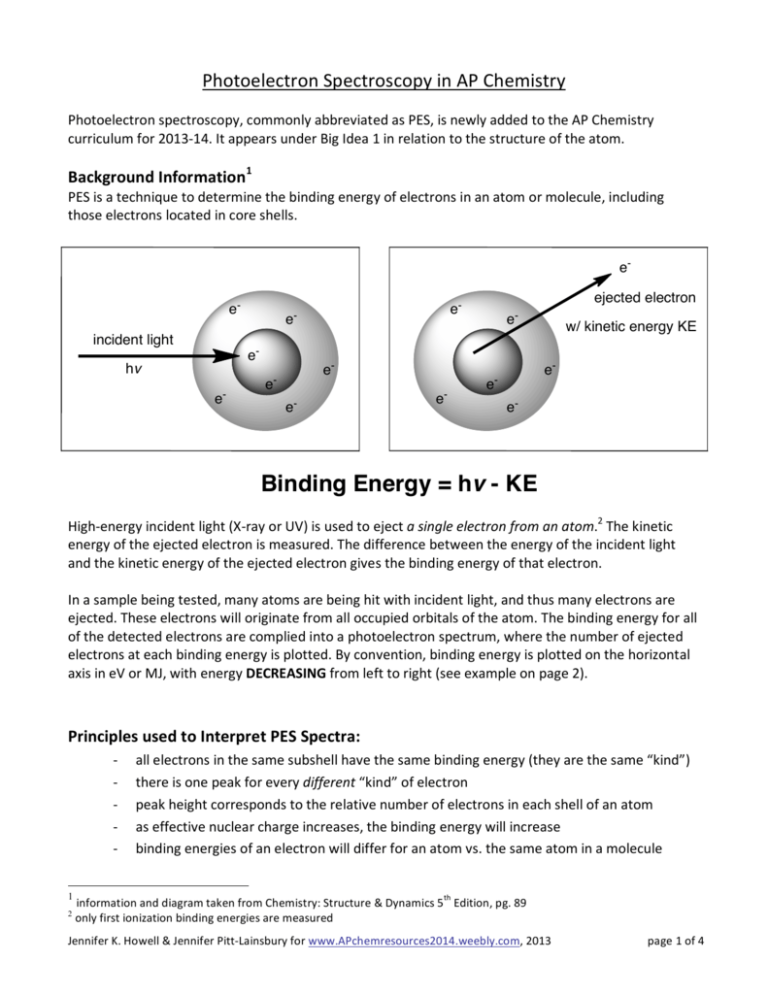
Photoelectron Spectroscopy in AP Chemistry Photoelectron spectroscopy, commonly abbreviated as PES, is newly added to the AP Chemistry curriculum for 2013-­‐14. It appears under Big Idea 1 in relation to the structure of the atom. Background Information1 PES is a technique to determine the binding energy of electrons in an atom or molecule, including those electrons located in core shells. eeincident light
e-
hv
e-
e-
e-
ejected electron
e-
e-
e-
e-
e-
w/ kinetic energy KE
e-
ee-
Binding Energy = hv - KE
High-­‐energy incident light (X-­‐ray or UV) is used to eject a single electron from an atom.2 The kinetic energy of the ejected electron is measured. The difference between the energy of the incident light and the kinetic energy of the ejected electron gives the binding energy of that electron. In a sample being tested, many atoms are being hit with incident light, and thus many electrons are ejected. These electrons will originate from all occupied orbitals of the atom. The binding energy for all of the detected electrons are complied into a photoelectron spectrum, where the number of ejected electrons at each binding energy is plotted. By convention, binding energy is plotted on the horizontal axis in eV or MJ, with energy DECREASING from left to right (see example on page 2). Principles used to Interpret PES Spectra: -­‐
-­‐
-­‐
-­‐
-­‐
1
2
all electrons in the same subshell have the same binding energy (they are the same “kind”) there is one peak for every different “kind” of electron peak height corresponds to the relative number of electrons in each shell of an atom as effective nuclear charge increases, the binding energy will increase binding energies of an electron will differ for an atom vs. the same atom in a molecule information and diagram taken from Chemistry: Structure & Dynamics 5th Edition, pg. 89 only first ionization binding energies are m easured
Jennifer K. Howell & Jennifer Pitt-­‐Lainsbury for www.APchemresources2014.weebly.com, 2013 page 1 of 4
Example: The following 3 questions refer to the photoelectron spectrum of neon as shown below: Relative Number
of Electrons
Photoelectron Spectrum of Neon
www.APchemresources2014.weebly.com
J. K. Howell
1000
C
A
B
800
600
400
200
Binding Energy (eV)
0
1. Peaks A, B, and C represent the binding energies of electrons in which subshells of neon? a. 1s, 2s, 2p c. 1s, 1s, 1s b. 2p, 2s, 1s d. 2s, 2p, 2p
2. Which of the following statements best accounts for peak A being far to the left of peaks B and C: a. the electron configuration of neon is 1s22s22p4 b. neon has 8 electrons located in its valence shell c. core electrons of an atom experience a much higher effective nuclear charge than valence electrons d. peaks B and C show first ionization energies of electrons in neon, whereas peak A shows the second ionization energy of neon electrons 3. Which of the following statements best accounts for peak C being three times the height of peak B: a. the intensity of the photoelectron signal at a given energy is a measure of the number of electrons in that energy level b. electrons represented by peak B have approximately triple the binding energy than those represented by peak C c. in a photoelectron spectrum, as binding energy increases the relative number of electrons decreases d. the height of peaks in a photoelectron spectrum does not have any relation to the structure of an atom Interpretation of Spectrum: The photoelectron spectrum of neon shows that the electrons in the 1s subshell (peak A) have a much higher binding energy (870 eV) than the electrons is the 2s subshell (peak B: 49 eV) and 2p subshell (peak C: 22 eV). Additionally, the 2p signal (peak C) is three times the height of the 1s and 2s signals (peak B), indicating that it contains triple the number of electrons (2p6 vs. 1s2 / 2s2). Answers: 1 (a), 2 (c), 3 (a) Sample Question: AP Chemistry Practice Exam and Notes, Fall 2013 #43 Jennifer K. Howell & Jennifer Pitt-­‐Lainsbury for www.APchemresources2014.weebly.com, 2013 page 2 of 4
PES and the Quantum Mechanical Model of the Atom: PES data demonstrates that energies of electrons in the same principle quantum number are not degenerate. This supports the existence of sub-­‐shells in the quantum mechanical model of the atom. The interpretation of PES data also gives information about: -­‐ number of subshells per principle quantum number and relative energies -­‐ number of electrons that can be held in each subshell -­‐ exceptions to the expected electron filling of subshells (copper, chromium) -­‐ which shells electrons are being removed from with the formation of ions (4s vs. 4d) How is PES incorporated into the AP Chemistry curriculum? There is a focus is on reading PES spectra and being able to draw conclusions based on the experimental observations. Additionally, the student should be able to interpret and relate various representations of the same data: electronic configurations, PES spectra and binding energies. !""#$%&'()*$+,(#-.#)/010/!"#$%"&'()"*+",()-(+%."(/"0%1&'*2%34",$&51%."%3%,'5(0+6"7$*,$",&0"3%&2%"'$%"&'()6"&0."&"
-(+*'*2%34",$&51%."08,3%8+"'$&'"*+")&.%"8-"(/"-5('(0+"&0."0%8'5(0+9"#$%"&''5&,'*(0"(/"'$%"%3%,'5(0+"'("'$%"08,3%8+"*+"'$%"
:&+*+"(/"'$%"+'58,'85%"(/"'$%"&'()9";(83():<+"3&7"*+"=8&3*'&'*2%34"8+%/83"/(5"80.%5+'&0.*01"'$%"+'58,'85%"(/"'$%"&'()9
-0">$('(%3%,'5(0"+-%,'5(+,(-4"?>@AB"-5(2*.%+"&"8+%/83")%&0+"'("%01&1%"+'8.%0'+"*0"'$%"8+%"(/"=8&0'8)")%,$&0*,+"'("
*0'%5-5%'"+-%,'5(+,(-*,".&'&"&0."%C'5&,'"*0/(5)&'*(0"(0"&'()*,"+'58,'85%"/5()"+8,$".&'&9"D0"-&5'*,83&56"3(7E5%+(38'*(0"
>@A"(/"&'()+"-5(2*.%+".*5%,'"%2*.%0,%"/(5"'$%"+$%33")(.%39"F*1$'",(0+*+'+"(/"-$('(0+6"%&,$"(/"7$*,$"$&+"%0%514"@"G"$!6"
7$%5%"$"*+">3&0,H<+",(0+'&0'"&0."!"*+"'$%"/5%=8%0,4"(/"'$%"3*1$'9"D0"'$%"-$('(%3%,'5*,"%//%,'6"*0,*.%0'"3*1$'"%I%,'+"
%3%,'5(0+"/5()"&")&'%5*&39"#$*+"5%=8*5%+"'$%"-$('(0"'("$&2%"+8//*,*%0'"%0%514"'("%I%,'"'$%"%3%,'5(09">$('(%3%,'5(0"
+-%,'5(+,(-4".%'%5)*0%+"'$%"%0%514"0%%.%."'("%I%,'"%3%,'5(0+"/5()"'$%")&'%5*&39"J%&+85%)%0'"(/"'$%+%"%0%51*%+"
-5(2*.%+"&")%'$(."'(".%.8,%"'$%"+$%33"+'58,'85%"(/"&0"&'()9"#$%"*0'%0+*'4"(/"'$%"-$('(%3%,'5(0"+*10&3"&'"&"1*2%0"
%0%514"*+"&")%&+85%"(/"'$%"08):%5"(/""%3%,'5(0+"*0"'$&'"%0%514"3%2%39
#0"#$%"%3%,'5(0*,"+'58,'85%"(/"&'()+"7*'$")83'*-3%"%3%,'5(0+",&0":%"*0/%55%."/5()"%2*.%0,%"-5(2*.%.":4">@A9"K(5"
*0+'&0,%6":('$"%3%,'5(0+"*0"L%"&5%"*.%0'*,&36"&0."'$%4"&5%":('$"5(81$34"'$%"+&)%".*+'&0,%"/5()"'$%"08,3%8+"&+"*0"L6"
7$*3%"'$%5%"&5%"'7("+$%33+"(/"%3%,'5(0+"*0"F*6"&0."'$%"(8'%5)(+'"%3%,'5(0"*+"/85'$%5"/5()"'$%"08,3%8+"'$&0"*0"L9
2#'3$&$.)456#7%&8#)/09"#$%"+'8.%0'"*+"&:3%"'(".%+,5*:%"'$%"%3%,'5(0*,"+'58,'85%"(/"'$%"&'()6"8+*01">@A".&'&6"*(0*M&'*(0"
%0%514".&'&6"&0.N(5";(83():O+"3&7"'(",(0+'58,'"%C-3&0&'*(0+"(/"$(7"'$%"%0%51*%+"(/"%3%,'5(0+"7*'$*0"+$%33+"*0"&'()+"2&549
:7&#$7#);3'7%&7#)/!"#$%"+'8.%0'",&0"8+%"5%-5%+%0'&'*(0+"&0.")(.%3+"'(",())80*,&'%"+,*%0'*/*,"-$%0()%0&"&0."+(32%"
+,*%0'*/*,"-5(:3%)+9
:;)/0<"#$%"+'8.%0'",&0""#$%&#'"#("#)#&*+*%,&)'+&-'.,-#/)'(/"0&'85&3"(5")&0E)&.%"-$%0()%0&"&0."+4+'%)+"*0"'$%"
.()&*0!".%+,5*:*01")(.*/*,&'*(0+"'("'$%"P($5")(.%3"'$&'"&5%"5%=8*5%.":4">@A".&'&9
:;)/0="#$%"+'8.%0'",&0""#0#1("#))'2#3'#/#.#&*)'(/"0&'85&3"-$%0()%0&"&,5(++")83'*-3%"5%-5%+%0'&'*(0+"*0"'$%".()&*0!"
5%&.*01"-$('(%3%,'5(0"+-%,'5(+,(-4".&'&"&0."'$%0"75*'*01"&0"%3%,'5(0",(0/*185&'*(0",(0+*+'%0'"7*'$"'$%".&'&6"(5"8+*01"
'$%"-%5*(.*,"'&:3%"'("-5%.*,'"%*'$%5"'$%"-$('(%3%,'5(0"+-%,'58)"(5"'$%"%3%,'5(0",(0/*185&'*(09
:7&#$7#);3'7%&7#)<!"#$%"+'8.%0'",&0"%01&1%"*0"+,*%0'*/*,"=8%+'*(0*01"'("%C'%0."'$*0H*01"(5"'("18*.%"*02%+'*1&'*(0+"7*'$*0"
'$%",(0'%C'"(/"'$%"Q>",(85+%9
:;)<0<"#$%"+'8.%0'",&0"#!+/4+*#')(#5%$%5'64#)*%,&)!"&5%">@A".&'&",(0+*+'%0'"7*'$"'$%"P($5")(.%36"(5",&0";(83():O+"3&7"
&0."'$%"+$%33")(.%3":%"8+%."'("%C-3&*0"7$4"'$%"*(0*M&'*(0"%0%514"(/"R&"*+"$*1$%5"'$&0"'$&'"(/"S:T
Jennifer K. Howell & Jennifer Pitt-­‐Lainsbury for www.apchemistryrevisions.weebly.com, 2013 page 3 of 4 The Photoelectric Effect: The Photoelectric Effect is the foundation for Photoelectron Spectroscopy. As the incident light increases in frequency, more electrons are ejected form the surface of the metal. The kinetic energy of the emitted electron only increases with increased energy of the incident light. This experimental evidence supports the theory that electrons in atoms are located in orbitals with discrete energy levels. Image f rom: http://hyperphysics.phy-­‐astr.gsu.edu/hbase/mod1.html Incident light (hv) greater than 1000 eV is considered X-­‐ray radiation, and the technique is sometimes called X-­‐Ray Photoelectron Spectroscopy (XPS). Incident light (hv) between 10 -­‐ 100 eV is considered to be in the vacuum ultraviolet spectrum, and the technique may be called Ultraviolet Photoelectron Spectroscopy (UPS). Resources: Spencer, James N., George M. Bodner, and Lyman H. Rickard. Chemistry: Structure and Dynamics. 5th Edition. New York: John Wiley & Sons. 2011. Pg. 88-­‐94 An AP Chemistry textbook approved by the College Board, which contains a comprehensive section on Photoelectron Spectroscopy. Ellis, Andrew, Miklos Feher, and Timothy Wright. Electronic and Photoelectron Spectroscopy: Fundamentals and Case Studies. Cambridge; 2005. Section 1.1 and Chapter 12 An in depth background on theory and variations for photoelectron spectroscopy. Diagrams of the spectrometer and details on the electron detection methods are provided. This resource is ideal for a teacher to gain background information on PES, but too in depth for student use. http://xdb.lbl.gov/Section1/Table_1-­‐1.pdf X-­‐Ray Data Booklet, by the Center for X-­‐ray Optics Advanced Light Source; 2001. A table of electron binding energies, in electron volts, for elements 1 -­‐ 92 in their natural form. Useful for designing test questions using authentic XPS data. Does not contain binding energies for valence electrons. http://www.colby.edu/chemistry/PChem/Lecture2.html -­‐ Go to “Atomic Orbital Ionization Energy” link Atomic Orbital Ionization Energies Table in eV, taken from “Electrons and Chemical Bonding,” Benjamin, 1964, Appendix
Jennifer K. Howell & Jennifer Pitt-­‐Lainsbury for www.apchemistryrevisions.weebly.com, 2013 page 4 of 4







