Solutions Exercises Lecture 4 II
advertisement
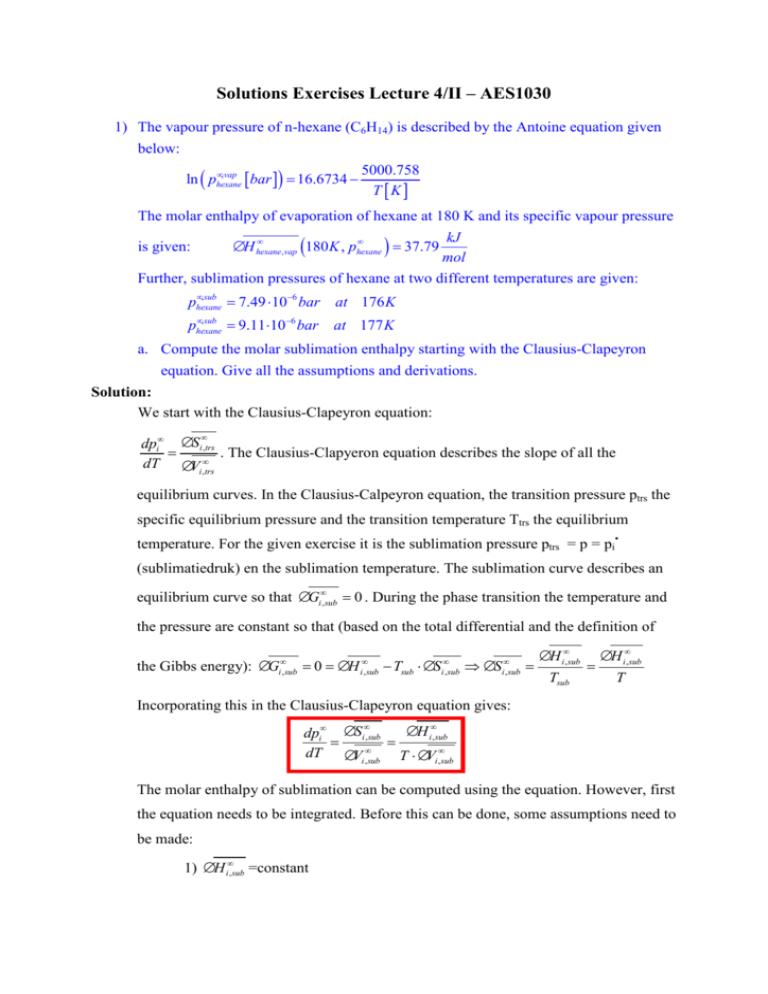
Solutions Exercises Lecture 4/II – AES1030 1) The vapour pressure of n-hexane (C6H14) is described by the Antoine equation given below: •, vap ln ( phexane [bar ]) = 16.6734 − 5000.758 T [K ] The molar enthalpy of evaporation of hexane at 180 K and its specific vapour pressure • • ∆ H hexane , vap (180 K , phexane ) = 37.79 kJ mol Further, sublimation pressures of hexane at two different temperatures are given: is given: • , sub phexane = 7.49 ⋅10−6 bar at 176 K • , sub phexane = 9.11⋅10−6 bar at 177 K a. Compute the molar sublimation enthalpy starting with the Clausius-Clapeyron equation. Give all the assumptions and derivations. Solution: We start with the Clausius-Clapeyron equation: • dpi• ∆ Si ,trs = . The Clausius-Clapyeron equation describes the slope of all the dT ∆Vi ,•trs equilibrium curves. In the Clausius-Calpeyron equation, the transition pressure ptrs the specific equilibrium pressure and the transition temperature Ttrs the equilibrium temperature. For the given exercise it is the sublimation pressure ptrs = p = pi• (sublimatiedruk) en the sublimation temperature. The sublimation curve describes an equilibrium curve so that ∆Gi•, sub = 0 . During the phase transition the temperature and the pressure are constant so that (based on the total differential and the definition of • i , sub the Gibbs energy): ∆G = 0 = ∆H • i , sub − Tsub ⋅ ∆ S • i , sub ⇒ ∆S • i , sub = ∆ H i•, sub Tsub = ∆ H i•, sub T Incorporating this in the Clausius-Clapeyron equation gives: • ∆ H i•, sub dpi• ∆ Si , sub = = dT ∆Vi •, sub T ⋅ ∆Vi ,•sub The molar enthalpy of sublimation can be computed using the equation. However, first the equation needs to be integrated. Before this can be done, some assumptions need to be made: 1) ∆ H i•, sub =constant 2) ∆Vi ,•sub = Vi ,•V − Vi •, S ≈ Vi ,•V because Vi ,•V ≫ Vi •, S 3) For not too high pressure (valid around the triple point) the molar volume of the gas phase can be described by the ideal gas law (note: here it is already accounted for that: Vi ,•V = R ⋅T pi• Incorporating this into the equation (with the red frame) and integrating gives the equation with which the molar enthapy of sublimation can be computed(note: instead of Tsub we write here T): ∆ H i•, sub ∆ H i•, sub ∆ H i•, sub ∆ H i•, sub ⋅ pi• dpi• = ≈ = = dT T ⋅ ∆Vi ,•sub T ⋅Vi ,•V T ⋅ R ⋅ T R ⋅T 2 • pi • dp • ∆ H i , sub dT ⇒ •i = ⋅ 2 ⇒ pi R T pi• (T2 ) T • dpi• ∆ H i , sub 2 dT = ⋅ ∫ pi• ∫ T2 R T1 p • (T ) pi• (T2 ) ∆ H i•, sub ⇒ ln • =− p (T ) R i 1 i 1 1 1 ⋅ − T2 T1 p • (T ) 1 ⇒ ∆ H i•, sub = − R ⋅ ln i• 2 ⋅ pi (T1 ) 1 − 1 T2 T1 With the given data (sublimation pressures at 176 K and 177 K) we can compute the molar enthalpy of sublimation: T1= 176 K and pi•(176 K) = 7.49·10-6 bar T2 = 177 K and pi•(177 K) = 9.11·10-6 bar • ∆ H hexane , sub = −8.314 9.11 ⋅10 −6 bar J 1 J kJ ⋅ ln = 50712.8 = 50.71 ⋅ −6 mol ⋅ K mol mol 7.49 ⋅10 bar 1 − 1 1 177 K 176 K b. By using the given data compute for hexane the triple temperature (in K) and the pressure of the triple point (in bar). Solution: The triple point is the point in the pressure, temperature phase diagram at which the three equilibrium curves, namely the vapour pressure curve, the melting curve and the sublimation curve, cross each other. Thus for the computation of the triple point temperature and pressure at least two of the equations for the description of the equilibrium curves need to be equalize with each other. Here the given equation for the vapour pressure curve and the in exercise a derived equation describing the sublimation curve are equalized with each other. Note that now in the equation for the sublimation curve the temperature T2 is the triple point temperature and the belonging pressure the triple point pressure; phexane• (Ttr). For T1 an arbitrary temperature and its belonging sublimation pressure can be used. However, the difference between this chosen temperature and the expected value for the triple point temperature should not be too large. • 1 ∆ H hexane 1 , sub • • ln phexane ln 176 T = p T = K − ⋅ − ( tr ) ) hexane ( 1 T T ( = 176 K ) R 1 tr J 50713 • mol ⋅ 1 − 1 ⇒ ln phexane (Ttr ) = ln 7.49 ⋅10−6 bar − J Ttr 176 K 8.314 mol ⋅ K 1 1 1 • ⇒ ln phexane (Ttr ) = −11.802 − 6099.71 ⋅ − K Ttr 176 K ( ) ( ( ) ( ) ) ( ) 1 1 ⋅ − Ttr 176 K ⇒ 5000.758 •, vap ln ( phexane (Ttr ) ) = 16.6734 − Ttr •, sub ln ( phexane (Ttr ) ) = −11.802 − 6099.71 1 K 1 1 5000.758 ⋅ − = 16.6734 − Ttr Ttr 176 K 6099.71 6099.71 5000.758 1 = ⋅ ( 6099.71 − 5000.758 ) ⇒ −11.802 − 16.6734 + = − 176 Ttr Ttr Ttr −11.802 − 6099.71 ⇒ 6.182 = 1 K 1098.95 1098.95 ⇒ Ttr = = 177.8 K 6.182 Ttr •, vap ln ( phexane ,tr ) = 16.6734 − 5000.758 = −11.45 ⇒ phexane ,tr = exp ( −11.54 ) = 10.65 ⋅10−6 bar 177.8 c. Compute the molar enthalpy of fusion at the triple point. Give all the assumptions and derivation you make for the computation. Solution: With the available data the molar enthalpy of fusion cannot be determined as the molar enthalpy of sublimation or molar enthalpy of evaporation. Firstly, there are no temperatures and belonging pressures on the melting curve given. Secondly, there is no equation of state given for the description of the molar volumes of the solid or the liquid phase. This the molar enthalpy of fusion needs to be computed from the molar enthalpy of evaporation and the molar enthalpy of sublimation. We remember, that the enthalpy is a state function. Thus, for the computation of the change of enthalpy it does not matter which path is chosen as long as the initial and end state are the same. . With this in mind, the molar enthalpy of fusion which describes the phase transition from solid to liquid can be computed at the triple point by the following process; first phase transition from solid to gas (sublimation) followed by the phase transition from gas to liquid (condensation = reverse evaporation. If we further assume that the molar enthalpy of sublimation, the molar enthalpy of evaporation and the molar heat fusion are constant, the molar enthalpy of fusion can be computed by: ∆ H i•,vap = −∆ H i•,cond ∆ H i•,vap = H i•,V − H i•, L • • • ∆ H i , sub = H i ,V − H i , S ⇒ ∆ H i•, fus = H i•, L − H i•, S = H i•,V − H i•, S − H i•,V − H i•, L = ∆ H i•, sub − ∆ H i•,vap ∆ H i•, fus = H i•, L − H i•, S ( ) Giving • • • ∆ H hexane , fus = ∆ H hexane , sub − ∆ H hexane ,vap = ( 50.71 − 37.79 ) kJ kJ = 12.92 mol mol d. Which assumptions do you need to make to allow the computation of the molar enthalpy of evaporation from the Clausius-Clapeyron equation and two different temperatures? Solution: 1) ∆ H i•,vap =constant (this is realistic for T < 0.7 Tc) 2) ∆Vi ,•vap = Vi ,•V − Vi ,•L ≈ Vi ,•V realistic because for not too high temperatures Vi ,•V ≫ Vi •, L 3) For not too high pressures, e.g. at the triple point, the molar volume of the gas can be described by the ideal gas law: Vi ,•V = R ⋅T pi• Incorporating these assumptions and equations into the Clausius-Clapeyron equation with T = Tvap gives: ∆ H i•,vap ∆ H i•,vap ∆ H i•,vap ∆ H i•,vap ⋅ pi• dpi• = ≈ = = dT T ⋅ ∆Vi ,•vap T ⋅ Vi ,•V T ⋅ R ⋅ T R ⋅T 2 pi• • dp • ∆ H i ,vap dT ⇒ •i = ⋅ 2 ⇒ pi R T pi• (T2 ) T • dpi• ∆ H i ,vap 2 dT = ⋅ ∫ pi• ∫ T2 R T1 p• (T ) i 1 pi• (T2 ) ∆ H i•,vap 1 1 ⇒ ln • =− ⋅ − p (T ) R T2 T1 i 1 ( ) ( ) ⇒ ln pi•,vap (T2 ) = ln pi•,vap (T1 ) − ∆ H i•,vap 1 1 ⋅ − R T2 T1 2) a) Determine the slope of the melting curve of water, dT/dP, at the melting point (T = 273 K and p = pi• = 1atm). The molar enthalpy of fusion of water at T=273 K and p = pi• = 1 atm is 6010 J mol-1. At the same temperature and pressure the molar • volume of fusion is ∆Vi , fus = -1.63 cm3 mol-1. b) Next, compute the melting temperature of water at 1000 atm. c) Which assumptions need to be made to allow the computation of the molar enthalpy of fusion with the given data? The following data are given: Tfus (p = 1atm) = 273 K ∆Vi •, fus (273K, 1atm) = -1.63 cm3 mol-1. ∆ H i•, fus (273K, 1atm) = 6010 J mol-1 Wanted: a) dT /dp at Tfus (p = 1atm) = 273 K b) Tfus (p = 1000 atm) Solution: Ad a) Before the slope of the melting curve can be determined, first the equation for the description of the melting curve needs be derived. The melting curve describes the equilibrium between the liquid and the solid phase. The equilibrium is described by the equaltiy of the molar Gibbs energy of the solid and of the liquid phase: • • Gwater , L ( T , p ) = Gwater , S ( T , p ) For small changes of the temperature and the pressure with the system always in equilibrium, we can also write: • • d Gwater , L ( T , p ) = dGwater , S ( T , p ) In general, the change of the molar Gibbs energy can be described by: G d Gi• ( T , p ) = d = Vi • ⋅ dp − Si• ⋅ dT ⇒ Vi ,•L ⋅ dp L − Si•, L ⋅ dT L = Vi ,•S ⋅ dp S − Si•, S ⋅ dT S n If the system changes its state in such a way that it always displays an equilibrium between the liquid and the solid state, the changes in the temperature and the pressure in the coexisting phases are the same: dpL = dpS = dp en dTL = dTS = dT and: ( ) ( ) Vi ,•L ⋅ dp − Si•, L ⋅ dT = Vi ,•S ⋅ dp − Si•, S ⋅ dT ⇒ Vi ,•L − Vi •, S ⋅ dp − Si•, L − Si•, S ⋅ dT = 0 ( ) ( ) ) ) ⇒ Vi ,•L − Vi ,•S ⋅ dp = Si•, L − Si•, S ⋅ dT ( ( Vi ,•L − Vi ,•S dT ⇒ = dp Si•, L − Si•, S The phase transition from solid (S) to liquid (L) is described so that the last equation can be rewritten: dT fus • dpwater (V = (S • water , L • − Vwater ,S • water , L • − S water ,S ) = ∆V ) ∆S • water , fus • water , fus . Note that in this equation it is already incorporated that the phase transition from solid to liquid is described; the transition pressure p is replaced by pwater• and the transition temperature T by Tfus. • • In the exercise ∆Vwater , fus is given but not ∆ S water , fus . The change of the molar entropy of fusion can be replaced by properties which can be determined experimentally: • On the melting curve the liquid and the solid phase is in equilbrium so that: ∆ Gwater , fus = 0 . The molar Gibbs energy of fusion describes an isothermal and isobaric process and can be described by: • • • ∆ Gwater , fus = ∆ H water , fus − T fus ⋅ ∆ S water , fus = 0 • • • ⇒ ∆ H water , fus = T fus ⋅ ∆ S water , fus ⇒ ∆ S water , fus = • ∆ H water , fus T fus Thus the molar entropy of fusion is replaced by the molar enthalpy of fusion (=molar heat of fusion) and the melting temperature: • • • dT fus ∆Vwater ∆Vwater T fus ⋅ ∆Vwater , fus , fus , fus = = = . • • • • dpwater ∆ S water , fus ∆ H water , fus ∆ H water , fus T fus The equation given above describes the slope of the melting curve. The slope at the melting temperature Tfus(1atm)= 273 K can be computed with the given data: dT fus • dpwater cm3 m3 273K ⋅ −1.63 273K ⋅ −1.63 ⋅10−6 T fus ⋅ ∆V mol mol = = = • J Nm ∆ H water , fus 6010 6010 mol mol K K K = −7.4 ⋅10−8 = −7.4 ⋅10−8 = −0.0075 N 1 atm atm 2 5 m 1.013 ⋅10 • water , fus Ad b) Wanted: Tfus (p = 1000 atm) In exercise a the equation for the description of the slope of the melting curve has been derived. Integration of this equation gives an expression with which the melting temperature • at 1000 atm can be computed. However, in order to do this we need to assume that ∆Vi , fus = • constant and ∆ H i , fus = constant…this is quite a rough assumption seeing that the pressure changes from 1 atm to 1000 atm. However, if this assumption is not made the computation of the melting temperature is not possible. So, we use these values but keep the assumption in mind: dT fus • dpwater ⇒ = • T fus ⋅ ∆Vwater , fus • ∆ H water , fus • T fus ( pwater ,2 ) • pwater ,2 ∫ ∫ • T fus ( pwater ,1 dT = T fus ) • pwater ,1 = • ∆Vwater , fus • ∆ H water , fus • ∆Vwater , fus ∆H • water , fus ⋅ T fus ⋅ dp = • ∆Vwater dT , fus ⇒ = ⋅ dp • T fus ∆ H water , fus • ∆Vwate r , fus ∆H • water , fus • pwater ,2 ⋅ ∫ • dpwater • pwater ,1 • • T fus ( pwater ∆Vwater ,2 ) , fus • • ⇒ ln = ⋅ ( pwater ,2 − pwater ,1 ) • • T fus ( pwater ,1 ) ∆ H water , fus ⇒ • T fus ( pwater ,2 ) • T fus ( pwater ,1 ) • ∆Vwater , fus • • = exp ⋅ ( pwater − p ) ,2 water ,1 ∆H • water , fus • ∆Vwater , fus • • • • ⇒ T fus ( pwater = T p ⋅ exp ⋅ p − p ( water ,2 water ,1 ) ,2 ) fus ( water ,1 ) ∆H • water , fus cm3 − 1.63 mol ⋅ (1000atm − 1atm ) ⇒ T fus ( p = 1000atm ) = 273K ⋅ exp 6010 J mol 3 −6 m − ⋅ 1.63 10 mol = 273K ⋅ exp Nm 6010 mol N N ⋅ 1000 ⋅1.013 ⋅105 2 − 1 ⋅1.013 ⋅105 2 m m T fus ( p = 1000atm ) = 265.61K (Note: Tfus(pi•) is the melting temperature at the pressure pi•) The melting temperature can also be determine in a different manner and is given below. For this approach it is assumed that the slope of the melting curve is constant over the complete pressure range. dT fus K = −0.0075 = co ns tan t • dpwater atm ( • T fus pwater ⇒ ∫ ) • T fus ( pwater ,0 ) dT = T fus ( p • water ) −T ( p fus • • T fus ( pwater ) = T fus ( pwater ,0 ) − 0.0075 p• • water ,0 water K K • • = − ⋅ ⋅ ( pwater − pwater 0.0075 dp = −0.0075 ) ,0 ) ∫ atm p• atm water ,0 K • • ⋅ ( pwater − pwa ter ,0 ) atm • • ⇒ T fus ( pwater = 1000atm ) = T fus ( pwater ,0 = 1atm ) − 0.0075 • T fus ( pwater = 1000atm ) = 273K − 0.0075 K ⋅ atm (( p K ⋅ 999atm = 265.5 K atm • water • = 1000atm ) − ( pwater ,0 = 1atm ) ) 3) The following (transition) pressures are given: ice water T [K] p = pi• [mmHg] transition 269.15 3.280 Sublimation 271.15 3.880 Sublimation 275.15 5.294 Evaporation 277.15 6.101 Evaporation First derive the necessary equations from the Clausius-Clapeyron equation and then Compute the following properties: a) Molar heat of sublimation b) Molar heat of evaporation c) Molar heat of fusion d) Triple point temperature and pressure Solution: Ad a) and b) Note: In the following text the derivations and computations are given for component i (indicated by the index i). For the given exercise this component I is water, i = H2O. For the derivation of the equations we start with the Clausius-Clapeyron equation: • dpi• ∆ Si ,trs = . The Clausius-Clapyeron equation describes the slope of all equilibrium dT ∆Vi ,•trs curveswith the transition pressure ptrs= p = pi• and the transition temperature Ttrs= T. On the equilibrium curves we know that ∆Gi•,trs = 0 . Further, we know that on the equilibrium curves we can state that T and p are constant so that: • i ,trs ∆G = 0 = ∆H Leading to: • i ,trs − Ttrs ⋅ ∆ S • i ,trs ⇒ ∆S • i ,trs = ∆ H i•,trs Ttrs = ∆ H i•,trs T • ∆ H i•,trs dpi• ∆ Si ,trs = = dT ∆Vi ,•trs T ⋅ ∆Vi ,•trs This equation is used for the computation of the molar enthalpy of sublimation and evaporation. Ad a) Before the molar enthalpy of sublimation can be computed using the above dervied equation, the equation needs to be rewritten. Therefore, a few assumptions need to be made: 1) ∆ H i•, sub =constant 2) ∆Vi ,•sub = Vi ,•V − Vi ,•S ≈ Vi ,•V because Vi ,•V ≫ Vi •, S 3) For not too high pressure, the molar volume of the gas phase is described by the ideal gas law: Vi ,•V = R ⋅T pi• With these assumptions we get (with T = Tsub): ∆ H i•, sub ∆ H i•, sub ∆ H i•, sub ∆ H i•, sub ⋅ pi• dpi• = ≈ = = dT T ⋅ ∆Vi ,•sub T ⋅Vi ,•V T ⋅ R ⋅ T R ⋅T 2 pi• • dpi• ∆ H i , sub dT ⇒ • = ⋅ 2 ⇒ pi R T pi• (T2 ) T • dpi• ∆ H i , sub 2 dT ∫ pi• = R ⋅ T∫ T 2 p • (T ) 1 p • (T ) ∆ H i•, sub ⇒ ln i• 2 = − p (T ) R i 1 ⇒ ∆H • i , sub i 1 1 1 ⋅ − T2 T1 pi• (T2 ) 1 = − R ⋅ ln • ⋅ p (T ) 1 1 i 1 − T2 T1 Using the given data: T1= 269.15 K and pi•(269.15 K) = 3.28 mmHg = 3.28 · 750 · 105 Pa T2 = 271.15 K and pi•(271.15 K) = 3.88 mmHg= 3.88 · 750 · 105 Pa We get: ∆ H i•, sub = −8.314 3.88mmHg J 1 J ⋅ ln = 50964.99 ⋅ 1 1 1 mol ⋅ K mol 3.28mmHg − 271.15 K 269.15 K Ad b) For the computation of the molar enthlpay of evaporation we make the following assumptions: 1) ∆ H i•,vap =constant (realistic for T < 0.7 Tc) 2) ∆Vi ,•vap = Vi ,•V − Vi ,•L ≈ Vi ,•V because Vi ,•V ≫ Vi ,•L 3) For not too high pressures the molar volume of the gas phase can be described by the ideal gas law: Vi ,•V = R ⋅T pi• Incorporating these assumptions in the derived equation gives (with T = Tvap): ∆ H i•,vap ∆ H i•,vap ∆ H i•,vap ∆ H i•,vap ⋅ pi• dpi• = ≈ = = dT T ⋅ ∆Vi ,•vap T ⋅ Vi ,•V T ⋅ R ⋅ T R ⋅T 2 pi• • dp • ∆ H i ,vap dT ⇒ •i = ⋅ 2 ⇒ pi R T pi• (T2 ) T • dpi• ∆ H i ,vap 2 dT = ⋅ ∫ pi• ∫ T2 R T1 p• (T ) i 1 pi• (T2 ) ∆ H i•,vap 1 1 ⇒ ln • =− ⋅ − p (T ) R T2 T1 i 1 pi• (T2 ) 1 • ⋅ ⇒ ∆ H i ,vap = − R ⋅ ln • p (T ) 1 1 i 1 − T2 T1 With the given data, the molar enthalpy of evaporation can be computed: T1= 275.15 K en pi•(275.15 K) = 5.294 mmHg= 5.294 · 750 ·105 Pa T2 = 277.15 K en pi•(277.15 K) = 6.101 mmHg = 6.101 · 750 ·105 Pa ∆ H i•,vap = −8.314 6.101mmHg J 1 J ⋅ ln = 44976.05 ⋅ 1 1 1 mol ⋅ K mol 5.294mmHg − 277.15 K 275.15 K Ad c) With the available data the computation of the molar enthalpy of fusion cannot be done as for the molar enthalpy of sublimation and of evaporation. The molar enthalpy of fusion is therefore determined from the molar enthalpy of evaporation and of sublimation. This is only possible because the molar enthalpy is a state function and thus the change of the molar enthalpy depends only on its initial and its end state. The phase transition from solid to liquid (fusion) is now split into two processes, namely first the phase transition from solid to gas followed by the phase transition from the gas to the liquid state. The latter process, the condensation, is the reverse process of evaporation. Again because the enthalpy is a sate function, we know that the enthalpy of evaporation is the equal to the negative enthalpy of condensation. If further it is assumed that all mola renthalpies of transition are constant, we get: ∆ H i•,vap = H i•,V − H i•, L • • • ∆ H i , sub = H i ,V − H i , S ⇒ ∆ H i•, fus = ∆ H i•, sub − ∆ H i•,vap ∆ H i•, fus = H i•, L − H i•, S J J J ∆ H i•, fus = 50964.99 − 44976.05 = 5988.94 mol mol mol Ad d) In this exercise it is asled to compute the triple point temperature and pressure. The triple point describes the phase equilibriuim between a liquid, a solid and a gas phase. Further it is the point at which the melting curve, the vapour pressure curve and the sublimation curve cross. By equalizing two of the equations describing the equilibrium curves with each other, the triple point temperature and pressure can be determined. In exercise a and b the equations for the description of the vapour pressure curve and of the sublimation curve have been derived and are now used to determine the triple point temperature and pressure. The vapour pressure at the triple point temperature is described by: ∆ H i•,vap 1 1 ln p (Ttr ) = ln p (T1 ) − ⋅ − R Ttr T1 For T1 an arbitrary temperature slightly larger than the expected triple point temperature is chosen. Thereby, the vapour pressure at this temperature should be known. For example we choose T1 = 275.15 K with pi•(275.15 K) = 5.294 mmHg (computed with the expression for the description of the vapour pressure curve). With the molar enthalpy of evaporation J ∆ H i•,vap = 44976.05 we have all data to allow the use of the expression mol given above. ( ) • i ( • i ) The same approach is used for the sublimation curve. The sublimation pressure at the triple point temperature is given by: ∆ H i•, sub 1 1 ln p (Ttr ) = ln p (T2 ) − ⋅ − R Ttr T2 With T2 a temperature slightly smaller than the expected triple point temperature, e.g. T2 = 269.15 K, and the belonging sublimation pressure pi•(269.15 K) = 3.28 mmHg and the molar enthalpy of sublimation J ∆ H i•, sub = 50964.99 we have all data to describe the sublimation curve mol completely. Equalizing the vapour pressure with the sublimation pressure curve at the triple point temperature gives: ∆ H i•,vap 1 1 • • ln pi (Ttr ) = ln pi (T1 ) − ⋅ − R Ttr T1 • ∆ H 1 1 i , sub ln pi• (Ttr ) = ln pi• (T2 ) − ⋅ − R Ttr T2 ( ) ( ( ) ( ) ( ) ( ) • i ( ⇒ ln p (T1 ) • i ) • i ) ∆ H i•,vap 1 1 ∆ H i•, sub 1 1 • − ⋅ − = ln pi (T2 ) − ⋅ − R R Ttr T1 Ttr T2 ( ) pi• (T1 ) ∆ H i•,vap ∆ H i•, sub 1 ⇒ ln • + − = p (T ) R ⋅ T R ⋅ T2 Ttr 1 i 2 ∆ H i•,vap ⇒ Ttr = ln ∆H • ∆ H i•, sub i ,vap ⋅ − R R ∆ H i•, sub − R R • • pi (T1 ) ∆ H i ,vap ∆ H i•, sub − + R ⋅ T1 R ⋅ T2 pi• (T2 ) Filling the given data into the equation allows the computatio of the triple point temperature: J J 50964.99 mol ⋅ K − mol ⋅ K J J 8.314 8.314 mol ⋅ K mol ⋅ K Ttr = J J 44976.05 50964.99 5.294mmHg mol ⋅ K mol ⋅ K ln + − J J 3.28mmHg 8.314 ⋅ 275.15 K 8.314 ⋅ 269.15 K mol ⋅ K mol ⋅ K Ttr = 273.28 K 44976.05 The triple point pressure can be computed by filling the determined triple point temperature into the equation either describing the sublimation pressure or the vapour pressure: ( ) ( ) ln pi• (Ttr ) = ln pi• (T2 ) − ( ) ( ) ln ptr = pi• ( 273.28 K ) ln ptr = pi• ( 273.28 K ) ∆ H i•, sub 1 1 ⋅ − R Ttr T2 J mol ⋅ K = ln ( 3.28mmHg ) − J 8.314 mol ⋅ K = 1.532 ⇒ ptr = pi• ( 273.28 K ) = 4.627 mmHg 50964.99 1 1 ⋅ − 273.28 K 269.15 K