Experiment # 12 - Organic Chemistry
advertisement
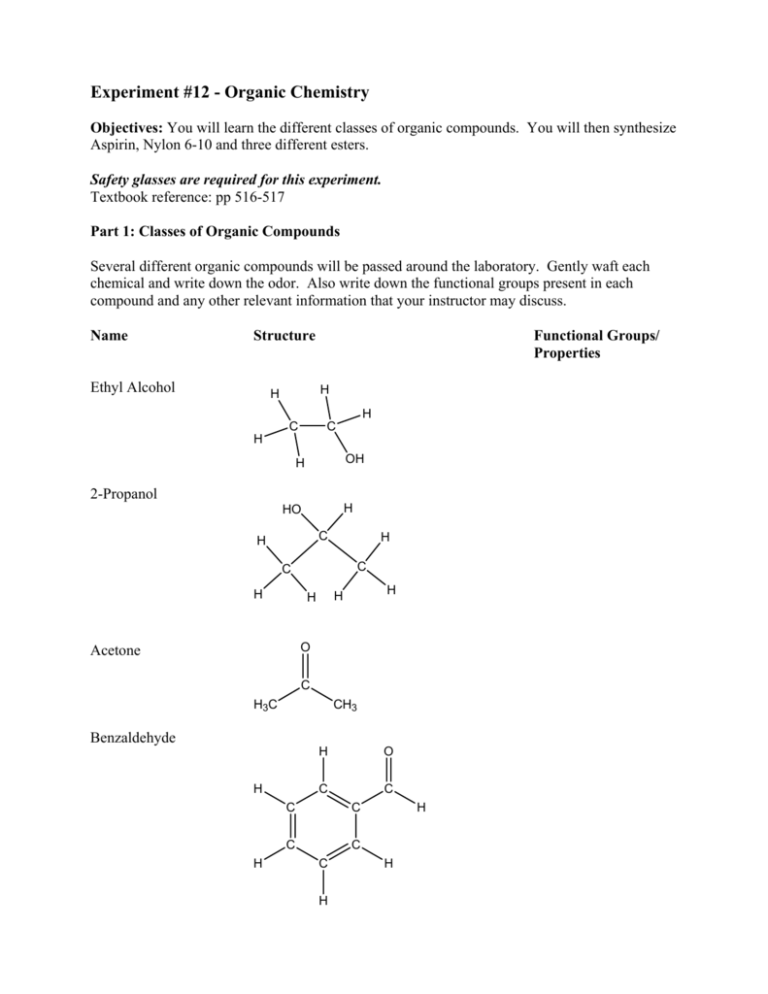
Experiment #12 - Organic Chemistry Objectives: You will learn the different classes of organic compounds. You will then synthesize Aspirin, Nylon 6-10 and three different esters. Safety glasses are required for this experiment. Textbook reference: pp 516-517 Part 1: Classes of Organic Compounds Several different organic compounds will be passed around the laboratory. Gently waft each chemical and write down the odor. Also write down the functional groups present in each compound and any other relevant information that your instructor may discuss. Name Structure Ethyl Alcohol Functional Groups/ Properties H H H C C H OH H 2-Propanol H HO C H H C C H H H H O Acetone C H3C CH3 Benzaldehyde H C H H O C C C C C C H H H Acetic Acid O C H3C OH Ethyl Acetate O H2 C C H3C CH3 O Amyl Acetate O H2 C C H3C H2 C O C H2 H Methyl Salicylate H C H2 O C C C C C H CH3 CH3 O C C OH H H3C Triethylamine CH2 H2C H3C N H2C CH3 2 Part 2: Preparation of Aspirin Aspirin has been widely used as analgesic (pain killer), antipyretic (fever-reducing) and antiinflammatory medicine. Its chemical name is acetylsalicylic acid (IUPAC name: 2(methoxycarbonyl)benzoic acid). In this experiment, you will use salicylic acid, acetic anhydride and phosphoric acid to synthesize aspirin. An excess amount of acetic anhydride will be used to enhance the percentage yield of aspirin. Carboxylic acid O O O O O OH + H3PO4 H3C O CH3 OH Salicylic acid OH + heat 70-80°C Acetic anhydride H3C O O CH3 OH Acetic Acid Ester Aspirin, an ester/acid Acetylsalicylic acid Safety Acetic anhydride and 85% phosphoric acid are highly corrosive. You must wear gloves and goggles while doing the experiment. Salicylic acid and aspirin may irritate skin and eyes. Do NOT breathe acetic anhydride vapor. This experiment should be done under good ventilation, especially for all transfers. Waste The chemicals used in this lab can be disposed of in the sinks with large amount of running water. Un-reacted acetic anhydride will be hydrolyzed to vinegar and 85% phosphoric acid will be highly diluted with running water. 3 Apparatus set-up for the experiment Erlenmeyer flask Buchner funnel beaker filter flask with side arm vacuum 5 4 6 5 7 8 3 2 9 2 1 11 6 4 3 7 8 hot plate 9 1 10 Apparatus for running reaction Apparatus for vacuum filtration Procedure 1. In a 125 ml Erlenmeyer flask, you will be given 2 g of salicylic acid. 2. Under a fume hood, add 6 ml of acetic anhydride to the Erlenmeyer flask with salicylic acid. Then, carefully add 6 drops of 85% phosphoric acid to the same flask. 3. Gently swirl your flask to make sure the reagents are well mixed. Place your Erlenmeyer flask in a 70 to 80 °C warm water bath on a hotplate. Keep the flask in the warm water bath for 20 to 30 minutes. 4. Remove the Erlenmeyer flask from water bath. 5. Add 40 mL of cold distilled water to your flask quickly. Then, place your flask in an icewater bath and swirl the flask. 6. Gradually, you should see some white crystals at the bottom of the flask placed in the icewater bath. If you do not see white solid or you observe some oil drops, use a stirring rod to scratch the inside wall of the flask to seed crystals while you use another hand to hold your flask in the ice-water bath. 7. After your product has crystallized, collect your aspirin crystals by vacuum filtration. While the vacuum is on, pour the mixture in the flask into the Buchner funnel. Wash your Erlenmeyer flask twice with some iced-water each time and transfer the water in your flask to the Buchner funnel. 4 Questions 1) Waft the aspirin crystals carefully, can you detect an odor? What is it? 2a) Which functional group in the salicylic acid compound was affected? 2b) What is the new functional group formed? 5 Part 3: Preparation of Nylon 6-10 by interfacial polymerization Introduction Nylons are polyamides synthesized by condensation polymerization of dicarboxylic acids or their derivatives (for example, diacyl chlorides) and diamines. Remarkable durability and flexibility give Nylons extensive applications as synthetic fibers. Nylon 6-10 is made by the interfacial condensation of 1,6-diaminohexane and sebacoyl chloride under basic conditions. The name of Nylon 6-10 is derived from the fact that it is prepared by 6-carbon diamine and 10carbon sebacoyl chloride, and the 6-carbon and 10-carbon units alternate and are separated by nitrogen atoms of the amide groups in the repeating unit of Nylon 6-10. O H H O N (CH2)6 N C (CH2)8 C O O H2N(CH2)6NH2 ClC (CH2)8 CCl 1,6-diaminohexane sebacoyl chloride n Nylon 6-10 O n H2N(CH2)6NH2 + O n ClC-(CH2)8-CCl NaOH, - 2n HCl H HO O N-(CH2)6-N-C-(CH2)8-C n Safety Sodium hydroxide is a strong base and highly corrosive. It can cause severe damage to skin and eyes. Hexane is a highly volatile organic solvent and must not be used near flame or heat. 1,6diaminohexane is irritating, corrosive and gives off toxic fumes upon being heated. Sebacoyl chloride is extremely harmful to skin, eyes and respiratory system. You must wear goggles and gloves when doing this experiment. This experiment must be conducted in well-ventilated fume hoods or rooms. 6 Procedure: 1. To a dry and clean 100-mL beaker, add 20 ml of 5% sebacoyl chloride solution. 2. Slowly add 20 ml of 5% 1,6-diaminohexane in 3 M NaOH solution to the beaker. 3. Let the reaction mixture stand still for one minute. You should see a white film formed at the interface of the two layers in the 100-mL beaker. 4. Dip forceps into the beaker and let it reach the interface of the two layers. Then, grasp the white nylon film at the interface area, slowly pull the polymer strand out of the solution and wrap the strand around the central part of a stirring rod. Hold the rod firmly and slowly wind up your nylon. If you do it very carefully and slowly, you should be able to obtain an unbroken nylon strand until one of your reactants is used up. If your nylon strand snaps, use forceps to generate a new strand. 5. Place the Nylon into a 250-mL water filled beaker. 6. Observe the physical appearance of your product. Test the tensile strength of your nylon strand by stretching until it breaks. Questions 1) Where did the reaction take place when the reagents were mixed? 2) Describe the appearance of your Nylon 6-10 product and the tensile strength of your nylon product (that is, qualitatively how much effort is needed to break the nylon strand). 3) What are some uses of Nylon? 4) Name two natural polymers? 5) Do we have any polymers in our body? Explain. 7 Part 4: Synthesis of Esters You will be synthesizing the three esters you observed in part 1 (ethyl acetate, amyl acetate and methyl salicylate) by a method called Fischer esterification. In this method, a carboxylic acid and an alcohol react in the presence of acid to yield an ester and water. A. O O + C H3C CH3CH2OH H3PO4 H3C OH acetic acid H2 C C ethanol + H2O + H2O CH3 O ethyl acetate B. O O C H3C + CH3(CH2)4OH H3PO4 OH C H3C acetic acid O(CH2)4CH3 amyl acetate amyl alcohol O O C. CH3 OH + CH3OH OH salicylic acid O H3PO4 + H2O OH methanol methyl salicylate Procedure: Add about 3 mL of the alcohol to the test tube that already contains the carboxylic acid. Then add 3 drops of phosphoric acid to the test tube and place the test tube in a hot water bath. Let the reaction proceed until the ester is formed (indicated by the odor you observed in part 1). 8