Chem 4563 Organic Qualitative Analysis Phenols, Enols and
advertisement

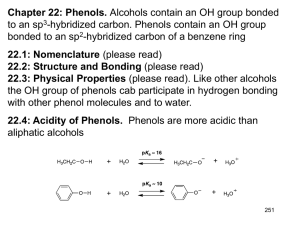
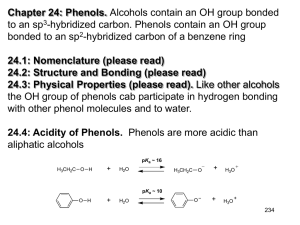
Chem 4563 Organic Qualitative Analysis Phenols, Enols and Carboxylic Acids-Functional Group Tests 1. Introduction Acidic compounds containing only carbon, hydrogen and oxygen are either phenols, enols or carboxylic acids. Phenols and enols are compounds of intermediate acidity between that of carboxylic acids and alcohols. Alcohols do not show acidic properties in aqueous medium, whereas both phenols, enols and carboxylic acids react with, and are soluble in 5% aqueous sodium hydroxide solution (exceptions are the highly hindered phenols). Acids and phenols may be differentiated on the basis of the insolubility of phenols in 5% aqueous sodium bicarbonate solution (with the exception of certain phenols substituted by several electron-withdrawing groups such as 2,4-dinitrophenol). The pKa of acids is about 5, whereas the pKa of phenols and enols is around 10. As a result acids are in solubility class A1(although certain low molecular weight or difunctional acids are in class SA), whereas phenols are in class A2. There are no good general functional group tests for the indication of a carboxylic acid. Usually the determination of solubility class A1 is sufficient to suggest an acid. Sulfonic acids may also be in class A1, but sulfonic acids also contain a sulfur atom which should be detected by the sodium fusion test results. In addition, certain substituted phenols such as dinitrophenol or trinitrophenol will show solubility in A1, but again, these compounds should give a positive test for nitrogen from the sodium fusion results. In the absence of sulfur or nitrogen...you probably have a carboxylic acid. 2. Tests for Phenols a Ferric Chloride Test Treatment of a 1% solution of FeCl3 in chloroform with a phenol yields a triaryloxy complex. This reagent is useful for detecting the presence of a hydroxy group attached directly to an aromatic nucleus, alcohols do not undergo this reaction Most phenols yield intense red, blue, purple or green colorations in the ferric chloride test . Other functional groups produce color changes with ferric chloride: aliphatic acids give a yellow solution, aromatic acids give a tan precipitate. OH H N pyridine + FeCl3 Fe O + Cl CHCl3 3 triaryloxy complex intensely colored All phenols do not produce color with this reagent. Highly hindered phenols (such as 2,6-di-tertbutyphenol) fail to give positive tests. A negative test must not be taken as confirming the absence of a phenol without additional supporting evidence. Enols give a distinctive yellow color when treated with FeCl3 /CHCl3 /pyridine. b. Bromine Water Test Phenols react rapidly with bromine water to produce insoluble substitution products; all available positions ortho and para to the phenol are brominated. OH OH Br + Br2 Br + H2O Br 3 HBr The bromine color is rapidly discharged and eventually the insoluble 2,4,6-tribromophenol will precipitate out. Water is used as the solvent instead of chloroform because the somewhat complex substitution mechanism procedes through an ionic intermediate, which is greatly stabilized through solvation with the water. This reaction can be used as both a classification test and as a derivitization procedure. Other highlyactivated aromatic compounds will also give bromine substitution. This test should be applied with some discrimination since aniline and alkoxy aryl ethers will also react rapidly with bromine wter to produce insoluble preciptates. Enols will react with bromine water to form a brominated aldehyde or ketone product which is also insoluble c. Phenolate Test(Uv/Visible Spectroscopy) One useful test for phenols is not exacly a classic wet chemistry test since it does involve some simple spectroscopy is the Phenolate test. In the ultraviolate region, ionization of a phenol by a base increases both the wavelength and the intensities of the absorption bands. OH O NaOH H+ λmax = 270 mµ λmax = 297 mµ Phenols with a high degree of conjugation in their conjugate base will give colored solutions. This shift may be observed visually in the case of phenols substituted by nitro or cyano groups at the ortho and/or para positions: p-nitrophenol is yellow, whereas a solution of sodium p-nitrophenolate is red. d. Tests for Enols Enols can be differentiated from phenols by the fact that enols can be oxidized by Jones Reagent. During this oxidation, the orange-red Cr (+6) ion is reduced to the greenish Cr(+3) ion. Phenols produce a dark-colored solution entirely unlike the characteristic green-blue color or a positive Jones Test. 3. Neutralization Equivalent of Acids One of the simplest and most ueseful ways to get information concerning a suspected carboxylic acid is to determine its neutralization equivalent.. A measured amount of the acid is titrated to a neutral end point by a standardized base. weight of acid sample in mg Neutralization Equivalent (N.E.) = volume of base in mL x molarity The neutralization equivalent of an acid or an acidic compound is its equivalent weight; the molecular weight may be determined from the neutralization equivalent by multiplying that value by the number of acid functional groups in the molecule. Since the pKa of both the organic acid and the indicator are sensitive to solvent changes, one should employ only enough ethanol to dissolve the organic acid. With high concentrations of ethanol, sharp endpoints are not employed with phenolphthalein as indicator. If it necessary to employ 95% or higher ethanol as solvent, bromothymol blue should be used as indictor. For accurate results, a blank should always be run on the solvent, and one should tke care that the neutralization equivalent is determined from a substance that is pure and anhdrous. Neutralization equivalents can be obtained with an accuracy of 1% or less.