Chemistry 101 Exam #3 Study Guide—Chapters 7-10
advertisement

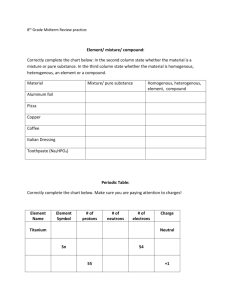
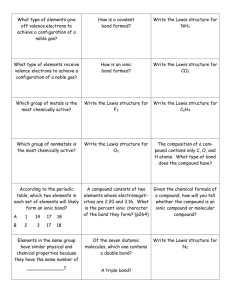
Chemistry 101 Exam #3 Study Guide—Chapters 7-10 Definitions to Know: Ionic compound Covalent compound Ionic bond Covalent bond Polarity Electronegativity Electron group Electronic geometry Molecular Geometry Enthalpy (H) Change in Enthalpy (∆H) Formal Charge Cation Anion Isoelectronic Ionization energy Photon Pauli Exclusion Principle Hund’s Rule Quantum numbers: n, l, ml, ms Be able to: Identify a covalent compound, ionic compound, covalent bond or an ionic bond Rank elements, bonds or compounds in terms of polarity Rank elements in terms of electronegativity Draw a Lewis dot diagram Draw a Lewis structure Identify the electronic geometry of a compound Identify the molecular geometry of a compound Identify the AXE notation of a central atom in a compound Calculate formal charge Calculate the change in enthalpy (∆H) of a chemical reaction given an unbalanced chemical reaction and the enthalpy values for different types of chemical bonds Write an electron configuration—know the two exceptions we discussed in class Write the Noble Gas configuration Determine relative ionization energies Determine relative size of atoms/ions Things to Study: Quiz #4 & #5 VSEPR Lab Lecture slides, chapters 9 & 10 homework problems Online review materials The following questions are practice for you to do. There is no guarantee that the exam will resemble these problems. 1. Calculate the wavelength, in nanometers, of an x-ray that has frequency of 5.15 × 1016 s−1. a. 5.83 b. 17.2 c. 0.172 d. 1.72 × 108 e. 583 Chemistry 101 Exam #3 Study Guide—Chapters 7-10 2. 3. 4. 5. 6. 7. 8. Answer: A An AM radio station operates at a frequency of 1080 kHz. What is the wavelength, in meters, of the radio waves? a. 360 b. 278 c. 1080 d. 3.60 e. 2.78 Answer: B Calculate the frequency, in s−1, of ultraviolet radiation that has a wavelength of 248 nm. a. 82.7 b. 248 c. 8.27 × 1015 d. 1.21 × 1015 e. 121 Answer: D Which of the following pairs is incorrectly matched? a. Wavelength - λ b. Frequency - f c. Mass - m d. Speed of light - c e. Planck's constant - h Answer: B For which of the following types of electromagnetic radiation would the photons have the greatest energy? a. x-rays b. UV c. visible d. IR e. microwaves Answer: A For which of the following types of electromagnetic radiation would the photons have the highest frequency? a. x-rays d. IR b. UV e. microwaves c. visible Answer: A Calculate the energy change, in joules, that occurs when an electron falls from the n = 4 to the n = 2 energy level in a hydrogen atom. a. −1.36 × 10−19 d. +4.09 × 10−19 −19 b. −4.09 × 10 e. −5.45 × 10−19 −19 c. +5.45 × 10 Answer: B Calculate the energy change, in joules, that occurs when an electron is raised from the n = 3 to the n = 5 energy level of a hydrogen atom. Chem 101 Study Guide Ch 7-10 Exam 3 9. 10. 11. 12. 13. 14. 15. 16. 17. a. 0.872 d. −2.42 b. −1.55 e. 2.42 × 10−19 −19 c. 1.55 × 10 Answer: C Select the species below that is not isoelectronic with the Ne atom? a. F− d. Mg2+ 2− b. O e. Ar c. Na+ Answer: E What is the ground state electron configuration of sulfur? a. [Ne] 2s22p4 d. [Ne] 3s23d4 2 4 b. [Ar] 2s 2p e. [Ar] 2p6 2 4 c. [Ne] 3s 3p Answer: C What is the ground state electron configuration of zinc? a. [Ar] 3s23d10 d. [Ar] 4s23d10 2 10 b. [Ar] 4s 4d e. [Kr] 3s24p10 2 6 2 10 c. [Ar] 3s 3p 4s 3d Answer: D Which of the following sets of elements is in the incorrect order of increasing atomic radius (smallest one first, etc.)? a. P, Si, Al d. Se, As, Sb b. Cl, Br, I e. I, Se, S c. S, As, Sn Answer: E Which of the following isoelectronic species would have the largest radius? a. Mg2+ d. F− + b. Na e. N3− 2− c. O Answer: E Which of the following sets of elements is arranged in the correct order of decreasing ionization energy (highest energy first, etc.)? a. Rb, K, Ca d. Cl, Br, I b. As, S, F e. Sb, Te, I c. K, Na, Li Answer: D Which set of elements is not in order of increasing atomic radius (smallest one first, etc.)? a. A) Na, K, Rb d. D) Br, Se, As b. B) F, S, As e. E) Be, Mg, Al c. C) C, N, O Answer: C Which set of elements is not in order of increasing atomic radius (smallest one first, etc.)? a. Ca2+, K+, S2− d. F, O, O2− + 2− 2− b. K , S , Se e. Na+, D−, F 3+ 2+ c. Fe , Fe , Fe Answer: E Which one of the following Lewis symbols is incorrect? A) B) C) D) E) Answer: C 18. A compound is found to form between As and Br. What is the most likely formula for this compound? a. AsBr d. AsBr3 b. As2Br e. As3Br c. As2Br3 Answer: D Chem 101 Study Guide Ch 7-10 Exam 3 19. Which of the following is the correct Lewis symbol for Br −? A) B) C) D) E) Answer: B 20. Which combination of atoms would be most likely to produce an ionic bond? a. Si, C d. C, H b. O, Cl e. S, Br c. Al, F Answer: C 21. Which is the least polar bond? a. O−Cl d. C−F b. S−F e. N−Cl c. P−Cl Answer: E 22. Which is the best Lewis structure for COI2? (Formal charges are not shown.) A) B) D) C) E) Answer: A 23. According to the Lewis theory, the total number of lone pair electrons in an NCl 3 molecule is a. 0. d. 9. b. 1. e. 10. c. 3. Answer: E 24. What is the formal charge on each oxygen in the figure below? a. b. c. −2, +1, 0 −1, −1, 0 0, 0, +3 d. +1, +1, 0 e. +2, −2, −2 Answer: B Chem 101 Study Guide Ch 7-10 Exam 3 25. What is the formal charge on nitrogen in the figure below? d. +1 a. −2 e. +2 b. −1 Answer: D c. 0 26. Estimate the ΔH°, in kJ, for the following reaction from the bond energies given. C3H8 (g) + Br2 (g) → C3H7Br (g) + HBr (g) Bond energy (kJ/mol): C–H, 414; C–Br, 276; Br–Br, 193; H–Br, 364. a. 33 d. −133 b. 133 e. 1247 c. −33 Answer: C 27. Estimate the ΔH°, in kJ, for the following reaction from the bond energies given. H2 (g) + I2 (g) → 2 HI (g) 28. 29. 30. 31. 32. 33. 34. Bond energy (kJ/mol): H–H, 436; H–-I, 297; I–I, 151. a. +7 d. +290 b. 884 e. −7 c. −290 Answer: E According to VSEPR theory, the shape of ICl4− is described as a. tetrahedral. d. octohedral. b. square planar. e. trigonal bipyramidal. c. square pyramidal. Answer: B Which of the following would not be linear? a. N2O d. SO2 b. CO2 e. CO c. I3− Answer: D What is the appropriate VSEPR notation for the central atom BCl3? a. AX2 d. AX3E b. AX2E e. AX2E2 c. AX3 Answer: C What is the appropriate VSEPR notation for the central atom H2S? a. AX2 d. AX2E2 b. AX2E e. AX4 c. AX3 Answer: D Which of the following molecules would be nonpolar? a. N2O d. H2O b. SCO e. CO2 c. HCCl3 Answer: E According to VSEPR theory, XeF2 has what molecular shape? a. linear d. bent b. tetrahedral e. trigonal bipryamidal c. trigonal pyramidal Answer: A According to VSEPR theory, XeF4 has what electron group geometry? a. tetrahedral d. see-saw b. trigonal pyramidal e. square planar c. octahedral Answer: C