9B. From Oil of Wintergreen to Salicylic Acid
Time: 2.5 hours
Required chemicals and solutions:
Reagent
Requirement/10 Students
Preparation of 1 L
Methylsalicylate
NaOH, 6 M
H2SO4, 8 M
40 mL
400 mL
200 mL
—
240 g of NaOH
444 mL of 18 M H2SO4
Other required materials:
Balance, platform or triple beam
Ice, as required
Filter paper for Büchner funnel, qualitative grade, 1student
Special note:
1. If the crystals of salicylic acid are dried in air for several days, the required time for
the experiment is only 2 hours. This time can also be achieved using an oven if the students work efficiently and effectively.
Connections: Early Greeks knew that the bark of willow trees could be used to reduce fever
and relieve pain. Later, chemists found that salicylic acid in the bark was responsible for
these curative effects.
Copyright © Houghton Mifflin Company. All rights reserved.
111
From Oil of Wintergreen to Salicylic Acid
Date:
........................................................................
Student name:
..................................................................................................
Course:
........................................................................
Team members:
..................................................................................................
Section:
........................................................................
..................................................................................................
Instructor:
........................................................................
..................................................................................................
Prelaboratory assignment
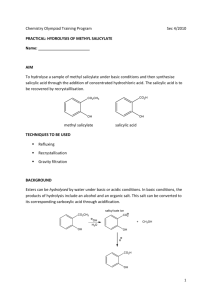
1. Draw sketches of methyl salicylate and salicylic acid, showing every
bond.
112
Copyright © Houghton Mifflin Company. All rights reserved.
From Oil of Wintergreen to Salicylic Acid
113
2. What is the limiting reactant in this experiment? Why? The density of
methyl salicylate is 1.18 gmL at 20C.
Molar mass of methyl salicylate 152.15 g/mol
Mol C8H8O3 4.0 mL 1.18 g/mL 1 mol
152.15 g
3.1 102 mol
Assume the other reactant is water in accord with
Figure 9B.1.
1 mol
Mol H2O 40 mL 1.0 g/mL 18.02 g
2.2 mol
Clearly, the limiting reactant is methyl salicylate.
3. What safety precautions must be observed during this experiment?
1. Be careful handling the solutions of sodium hydroxide
and sulfuric acid.
2. Do not touch the iron ring or wire gauze during
heating.
3. Make sure that the gas outlets are closed.
4. Do not ingest the sample of salicylic acid.
Copyright © Houghton Mifflin Company. All rights reserved.
From Oil of Wintergreen to Salicylic Acid
Date:
........................................................................
Student name:
..................................................................................................
Course:
........................................................................
Team members:
..................................................................................................
Section:
........................................................................
..................................................................................................
Instructor:
........................................................................
..................................................................................................
Results
43.9
Mass of methyl salicylate and graduated cylinder (g):
......................................
Mass of graduated cylinder (g):
......................................
Mass of methyl salicylate (g):
......................................
39.4
4.5
Description of crystals of salicylic acid:
White needles
106.5*
Mass of salicylic acid and beaker (g):
......................................
Mass of beaker (g):
......................................
Mass of salicylic acid (g):
......................................
102.9
3.6
*After sitting in air for three days.
Questions
1. a. What is the actual yield of salicylic acid?
The actual yield is 3.6 g.
b. Calculate the theoretical yield. Remember that you deduced the
limiting reactant in the Prelaboratory assignment.
The molar mass of methyl salicylate (C8H8O3) is 152.15 g/mol. The
molar mass of salicylic acid (C7H6O3) is 138.12 g/mol.
1 mol C8H8O3
1 mol C7H6O3
Theoretical yield 4.5 g C8H8O3 152.15 g C8H8O3
1 mol C8H8O3
114
138.12 g C7H6O3
1 mol C7H6O3
4.1 g
Copyright © Houghton Mifflin Company. All rights reserved.
From Oil of Wintergreen to Salicylic Acid
c.
115
Calculate the percentage yield.
% yield 3.6 g
4.1 g
100 88%
(Yields are typically greater than 80%.)
2. What covalent bonds in methyl salicylate and water were broken during
the reaction? What bonds were formed?
Copyright © Houghton Mifflin Company. All rights reserved.