Experiment 12 - Mr-Paullers-wiki
advertisement

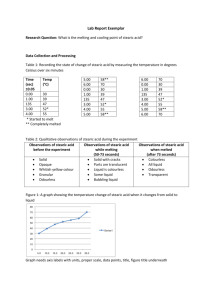
Approximating Avogadro’s Number Problem: Approximate the size of a stearic acid molecule using several simple measurements and the number of stearic acid molecules in a mole of stearic acid. Introduction: Stearic acid is a molecule with a long, hydrophobic hydrocarbon chain which contains a hydrophilic carboxylic acid (COOH) functional group on one end. When stearic acid solution is placed dropwise onto water, the stearic acid molecules will arrange so that the carboxylic functional group clings to the water and the hydrocarbon chain is raised straight above the carboxylic group. We will assume that the stearic acid layer will be a single molecule in depth and that stearic acid molecules are cubic. Stearic Acid: C18H36O2 A = πr2 V = πr2 h V = Ah A=h2 Materials: Large plastic tray meter stick lycopodium(baby) powder Stearic acid in hexane (0.12-0.15 g/L) – exact value will be provided in class Procedure: 1. Obtain a large plastic tray. Thoroughly clean the tray with soap and water and then rinse well. 2. Fill the tray with water to a depth of at least 1cm in the center of the tray. Using a shaker bottle, dust the water evenly with just enough lycopodium powder to make a visible layer. 3. Obtain a dropper and a 3.5 mL sample of stearic acid. 4. Being careful not to disturb the tray, add one drop of stearic acid to the center of the tray. Very quickly measure the diameter of the stearic acid monolayer by using a meter stick. Make four measurements with the meter stick held in a different orientation for each measurement. 5. Discard the contents of the tray and clean and rinse the tray. Repeat the experiment two more times. 6. Clean the tray. 7. Find the number of drops of stearic acid needed to dispense a volume of 1.00mL. Repeat this procedure three times. Data: Create a data table which will allow you to report all measurements made to complete the experiment. The data table should include all units, measurement uncertainty and the average value for the diameter of the stearic acid monolayer and the average number of drops of stearic acid in 1.00mL. Analysis and Conclusions: 1. Calculate the molar mass of stearic acid. 2. Determine the average volume of the stearic acid monolayer by using information determined in step 7 of the procedure. 3. Calculate the average area of the stearic acid monolayer by using the data collected in steps 4-5. 4. Calculate the height of the stearic acid film. Remember that 1mL = 1cm3 5. Using the assumption that stearic acid is cubic, calculate the area occupied by one molecule of stearic acid. 6. Calculate the mass of stearic acid that would be present in an average droplet of stearic acid solution. 7. Calculate how many molecules were present in the stearic acid monolayer (one drop). 8. Convert the mass of stearic acid in one droplet into moles. 9. Calculate your approximation of Avogadro’s number to determine how many molecules of stearic acid there are per mole. Use results from #7 and #8. 10. Determine the percent error of your experimental approximation of Avogadro’s number. 11. Identify sources of error and discuss their significance, i.e. will they cause your experimental value for Avogadro’s number to be higher or lower than the accepted 6.022 x 1023 and why? Name_________________________ Individual Pre-Lab Questions – Answer all questions in your own words! 1. Google “MSDS Stearic Acid” and “MSDS Cyclohexane,” what safety concerns are associated with these chemicals and what guidelines should you follow when completing this lab? 2. Calculate the molar mass of stearic acid C18H36O2. 3. How will you use your experimental data to calculate the average volume of the stearic acid? 4. Describe how you will calculate the area of the stearic acid monolayer or provide a formula. 5. How will determine the height of the stearic acid molecules or provide a formula. 6. How will you be able to approximate Avogadro’s number using your data?