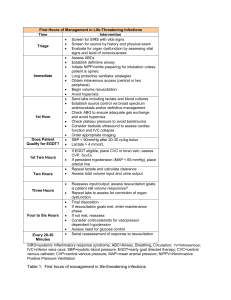
The Role of Hypotheses in Current Research, Illustrated by
advertisement

Journal of Cerebral Blood Flow & Metabolism 24:1235–1239 © 2004 The International Society for Cerebral Blood Flow and Metabolism Published by Lippincott Williams & Wilkins, Baltimore Commentary The Role of Hypotheses in Current Research, Illustrated by Hypotheses on the Possible Role of Astrocytes in Energy Metabolism and Cerebral Blood Flow: From Newton to Now Harold K. Kimelberg Neural and Vascular Biology Theme, Ordway Research Institute, Albany, New York Scientists currently strive to obtain reliable data on the properties of astrocytes in situ, and pari passu to define what the term astrocyte should encompass. As is the current fashion, they are also required to propose in a formal sense hypotheses for astrocytic function that serve as guides for such research. It has become the fashion to raise up “hypothesis-driven” science and denigrate descriptive science that lacks this formal introduction as merely cataloguing. Terms such as “fishing expedition” are often used pejoratively to describe scientific studies that are viewed as simply collecting data with no end in view. This seems as though it would be rather rare and would anyway describe the efforts of a rather inexperienced angler who does not have sufficient experience and skill to determine the types of fish that are likely to be caught where he or she is planning to fish. There is no dispute as to whether the postulation of hypotheses will lead to experiments. The real question, however, is whether always starting with hypotheses leads to more discoveries and a better-organized, more reliable database. To be consistent, this would have to be resolved scientifically; to test this hypothesis in the usual carefully controlled fashion with defined measures of whether so called hypothesis-driven science provides better data or more insights than so called non– hypothesis-driven science. It would be a simple exercise for the research professional to restate this question in the form of a hypothesis and develop a research plan to address it. To put it into practice, however, will rapidly lead to complications. For example, we would first need to define what non–hypothesis-driven science actually is and how it differs from the hypothesis-driven science. The lack of a formal hypothesis does not mean there is not some end in view. It may simply be that in the hypothesis-driven variety we take a positive view that we can guess pretty well what the mechanism or relation is, whereas in the latter case, we leave the question open and will come to this later because we believe we don’t have enough data to form a sensible hypothesis. I would hope that an individual scientist’s opinion, as an expert in his or her field on this issue, would be respected and not dismissed out of hand. You obviously need a background of reliable observations (that is, facts) to propose a hypothesis, and it may well not be clear to another observer when you are ready to propose it. The criticism on a case-by-case basis would be that any reasonable scientist, given the state of knowledge, should be able to come up with a reasonable hypothesis for the area of study under question. This is, in practice, unpredictable and when successful is referred to as scientific intuition. I will assume that everyone agrees that randomly obtained data, however reliable, with no question in mind (although by chance it may come up with important data leading to insights) is not acceptable because it is simply too much of a gamble, and this leaves one very uncomfortable. The two astrocyte-related hypotheses that I will cover to illustrate the problems of this common current practice are as follows: 1) that glucose enters the CNS via the astrocytic processes where it is converted by aerobic glycolysis to lactate which then serves as the principle source of energy for neurons (Magistretti et al., 1994; Pellerin and Magistretti, 2003; Voutsinos-Porche et al., 2003), and 2) that the processes of astrocytes that surround blood vessels at the levels of the arterioles affect smooth muscle contractility and therefore blood flow by releasing vasoactive agents. (Anderson and Nedergaard, 2003; Simard et al., 2003; Zonta et al., 2002). In a recent commentary regarding their well-known astrocyte-neuron lactate shuttle hypothesis (ANLSH), Received January 8, 2004; accepted June 9, 2004. Address correspondence and reprint requests to Dr. Kimelberg, Neural and Vascular Biology Theme, Ordway Research Institute, 150 New Scotland Ave., Albany, NY 12208; e-mail: hkimelberg@ ordwayresearch.org 1235 DOI: 10.1097/01.WCB.0000138668.10058.8C 1236 H. KIMELBERG Pellerin and Magistretti (2003) write, “The important point here is not so much to decide, based upon the actual pieces of evidence, whether an hypothesis is right or wrong but rather to point out what is heuristically valid in it, what we have learned, what remains to be assessed, what new hypotheses can be proposed, and which experiments are critical for this.” An alternative viewpoint was written some 330 years ago by Isaac Newton: “For the best and safest method of philosophizing seems to be, first to enquire diligently into the properties of things, and establishing those properties by experiments and then proceed more slowly to hypotheses for explanations of them. For hypotheses should be subservient only in explaining the properties of things, but not answered in determining them; unless so far as they may furnish experiments. For if the possibility of hypotheses is to be the test of truth and reality of things, I see not how certainty can be obtained in any science” (Christianson, 1984). Now, Newton was responding to criticisms by Ignance Gaston Pardies, a French Jesuit and professor of Rhetoric at the Collège de Louis-le-Grand in Paris, of Newton’s work with prisms that demonstrated that the simplest explanation of the well-known visible spectrum is that white light is a mixture of primary colors with different refractions. This is clearly a hypothesis in the best sense of the term that has been raised to the rank of a theory because it has been repeatedly shown to be a valid explanation of all sorts of optical phenomena, with now, of course, a much deeper understanding of what is going on. This is very different from the astrocyte-neuron lactate shuttle hypothesis where things are very much up in the air, as Pellerin and Magistretti do point out. But the question that I am addressing is why there is a modern insistence on the supremacy of hypotheses compared with Newton consigning them to a subsidiary role. Has the philosophical basis of the scientific method changed? Clearly the astrocyte-neuron lactate shuttle hypothesis arose from previous work. Pellerin and Magistretti thought these were sufficient to codify them into an hypothesis in 1994. But why did they propose a hypothesis? Did it help their thinking? If so, then it was a personal tool that did not need publicizing at that time when the evidence was (and still is) inconclusive. Did it lead to more experiments? Indeed yes, so for this aspect of Newton’s statement it was a good thing. But the authors could simply have said we need to know more about how glucose is handled by the brain. It has to enter from the blood and will therefore first encounter an astrocytic process, so we will study how glucose is handled by astrocytes. Wouldn’t the result be the same? The point I would like to make is that hypotheses tend to lock us into an either/or situation. This is the concern of the quote from Pellerin and Magistretti. Namely, that their hypothesis was never intended to be either/or. However, readers will tend to accept the hypothesis as stated withJ Cereb Blood Flow Metab, Vol. 24, No. 11, 2004 out the caveats. After all, if there are caveats, then it is not a very precise hypothesis and is difficult to refute experimentally, which, as Karl Popper has pointed out, is logically all you can do with hypotheses anyway (Lindh, 1993). So why hypotheses? They can definitely suggest new experiments, often clarify thinking, and make easy targets for grant reviews. However, Newton said that they should not determine anything, and this is particularly so for the merit of any proposed research whose usefulness can, a priori, only be guessed at. Hypotheses also clarify, as Newton fully realized: “And therefore because I have observed the heads of some great virtuoso’s to run much upon Hypotheses, as if my discourses wanted an Hypothesis to explain them by, & found, that some when I could not make them take my meaning when I spake of the nature of light & colors abstractly, have readily apprehended it when I illustrated my Discourse by an Hypothesis” (Christianson, 1984). The underlying observation for both hypotheses 1 and 2, which have been known since the times of Golgi (1885) and Ramón y Cajal (1913), is that the blood vessels in the central nervous system are surrounded by astrocytic processes, and we now know that this can be close to 100% (Virgintino et al., 1997). This fundamental fact has given rise to many hypotheses, ranging from development of the blood-brain barrier because of signals derived from the astrocytes as the central nervous system develops to the ASLNH hypothesis. It seems that all material that does not diffuse between the astrocytic processes will have to pass through the thin astrocytic processes or diffuse through the entire astrocyte. Now the spaces between the astrocytic processes are of the order of a few hundreds of angstroms wide. They could still form a major short circuit path as the membranes would be a major resistance pathway for polar substances unless there are sufficient carriers on the astrocytic processes, such as the Glu-1 carrier. There is also the question of why there are a high density of high affinity Glu-3 transporters on neuronal membranes (Leino et al., 1997) if the sequence is as follows: Glucose → endothelial cells → astrocytes → lactate → neuron Pellerin and Magistretti (2003) point out that this sequence is not obligatory, and parallel pathways can coexist, but then it will be very difficult to test this hypothesis because there are no limits to the degree to which deviations may be explained away. The proximity of astrocytic processes surrounding blood vessels also suggests a role in the control of blood flow (Anderson and Nedergaard, 2003; Zonta et al., 2002). For this, it is necessary for the control to be exerted at the level of the arterioles because the capillaries COMMENTARY: THE ROLE OF HYPOTHESES IN CURRENT RESEARCH lack the smooth muscle needed to cause contraction or relaxation to change the blood vessel diameter (Edvinsson et al., 1993; Traystman 1997). Note here that there must always be some basic tone to keep the arterioles at an intermediate diameter from which they can constrict or relax (Traystman, 1997). This hypothesis seems to be on firmer ground than the ASNLH. Indeed, Mulligan and MacVicar (2004) reported that Ca2+ in perivascular astrocyte processes leads to constriction of contiguous small arterioles via release of arachidonic acid. The astrocytic ensheathment of central nervous system blood vessels originates as blood vessels penetrate the brain parenchyma early in development from the arachnoid to the brain parenchyma and carry with them the glia limitans that are astrocytic processes. Interactions between the astrocytic sheath and the vascular endothelial cells are thought to be responsible for the formation of the interendothelial tight junctions that form the blood-brain barrier, which occurs at around the end of the first trimester in man (Abbott, 2002). However, if the role of the astrocyte is purely developmental, then why does the vascular ensheathment persist through adulthood? Presumably, they are then converted to physiologic roles or a constant astrocytic influence is needed to maintain the blood-brain barrier. Clearly, the former can be the ASLNH (Magistretti et al., 1994), pH control (Tschirgi, 1958), control of ingress of compounds such as glucose and amino acids or egress of waste metabolites (Pardridge, 1997), and control of [K+]o and blood flow (Newman, 1987; Zonta et al, 2002). These theoretical possibilities need to devolve into specific testable proposals that can be labeled as hypotheses, for the sine qua non of hypotheses, whether you are not too enamored of them or you cannot work without them, is that they have to be testable and that means in a direct and relevant manner and system. Evidence that there are the right lactate transporters (Allaman et al., 2000; Pellerin et al., 1998) is clearly only circumstantial and also needs to be shown in situ. Although cyclooxygenase-dependent products have been shown to be involved in vascular dilation in slices when astrocytes are directly stimulated (Zonta et al., 2002), there is no evidence that either COX1 or 2 are present in astrocytes in situ (Hoozemans et al., 2001). However, there are alternative pathways by which the astrocytic endfeet can influence the arterioles and the cyclooxygenase may be located elsewhere with the astrocytic influence being indirect, to explain the findings of Zonta et al. (2002). In general, evidence is very weak or perhaps even misleading when it only derives from astrocyte cultures. Differences in activities and protein profiles have been shown for primary astrocyte cultures as compared with freshly isolated GFAP(+) astrocytes (Kimelberg et al., 1997), or for Muller cells with increasing time in culture (Hauck et al., 2003). Thus it is a major weakness when 1237 both the original ASLNH hypothesis and the recent detailed rebuttal (Chih and Roberts, 2003) both use data for astrocytic and neuronal cultures to support and refute. The expression of the genome is plastic and influenced to a major degree by changes in the cellular environments via surface receptors (Peng et al., 1998). The latter follows logically from modern understanding of the biology of gene expression. Hypothetically, one reason why astrocytes may be developmentally retained to modulate arteriole diameter and therefore blood flow to respond to the varying energy needs of changes in brain activity is illustrated in Fig. 1. One should acknowledge, however, that this is only one possible hypothesis among many that can be proposed. Astrocytes are known to have a variety of receptors in situ as well as in vitro (Kimelberg, 1988, 1995; Porter and McCarthy, 1997) that can then integrate messages from neurons in the form of released transmitters so that the results of a wide range of different brain activities can be integrated and relayed to the blood vessels. In terms of the restraint, I propose that this is a good example of a reasonable hypothesis that one would have trouble supporting meaningfully. The existence of several receptors subtypes on freshly isolated astrocytes (Zhu and Kimelberg, 2004) is clearly consistent but not in any meaningful way because there could be numerous reasons for this coexistence. One possible approach that comes to mind is to determine whether the perivascular astrocytes are a specific subtype that can be specifically manipulated to see if it affects blood flow response to brain activity. This assumes the conclusion, as do all FIG. 1. Models comparing the hypothetical control of arteriolar diameter directly by individual neurons (A), with integration of inputs from different neurons through the perivascular astrocytic end feet (B). (A) The integration would be at the level of the arteriole or more strictly the smooth muscle cell, which would then have to integrate the different direct neuronal inputs. (B) The astrocyte acts as the integrator that translates the neuronal inputs for its receptors into an appropriate transmitter output for contraction or relaxation (black arrow). There is no obvious structure additional to the smooth muscle within the blood vessel that can serve this integrator role. J Cereb Blood Flow Metab, Vol. 24, No. 11, 2004 1238 H. KIMELBERG hypotheses, that there are vascular specific types, and this should be a doable task. After this is achieved, then one could talk about specific manipulation. It is not clear if all astrocytes have processes that surround blood vessels, although there is current evidence for the functional heterogeneity of astrocytes (Nedergaard et al., 2003; Zhou and Kimelberg, 2001). Note, though, that the demonstration of a specific perivascular astrocyte would have important implications for the ANLSH, as well as a host of other possible hypotheses. So, again, why can’t we state that our objective is to look for this cell? Then, the discussion would be only about whether proposed methods are likely to be successful, which is less a matter of opinion than the worth of some hypothesis. Direct evidence in situ is lacking for the ASNLH. The 1:1 relation between glucose uptake and glutamate turnover (Sibson et al., 1998) is consistent with a number of possible models and therefore is not definitive because other scenarios can be proposed to explain this relationship (Chih and Roberts, 2003). However, definitive tests of hypotheses regarding processes in organisms need to be obtained in vivo and that is why genetic knockouts are increasingly used. Such manipulations will need to be astrocyte-specific and possibly astrocytic subtypespecific, such as for the putative perivascular astrocyte just discussed. Thus the relation shown in Fig. 1 may only apply to a subgroup of astrocytes. Knockout experiments could be designed to see if 1) the elimination of the perivascular astrocytic glucose transporter essentially starves neurons, and 2) if, after knockout of astrocytic COX enzymes, the brain loses blood flow regulation. It will needed to be assessed if the knockouts are specific enough and then to see if the results are amenable of a clear interpretation. As always, it is better to conduct some pilot experiments, but, because of their complexity and the time needed to generate the animals, knockouts are not traditional pilot experiments. A hypothesis is only the first step in a laborious scientific investigation. Thus, as Newton wrote, hypotheses are a guide for experiments, but perhaps to be consistent with the scientific method we need only the publication of hypotheses supported by data. However, supporting data for the ANSLH are almost exclusively experiments with primary GFAP(+) astrocyte cultures. Because these cells have active glutamate transporters and, of course, the ubiquitous Na pump, the cells will naturally take up glutamate with Na, the intracellular accumulation of which then conventionally activates the pump. There will then be an increased uptake of glucose and, being adapted to a limited supply of oxygen, relative to consumption, through unstirred layers, the monolayer cultures are likely to mainly metabolize the glucose glycolytically with lactate production. The question is whether this occurs under the conditions that normally pertain in vivo. The ANLSH seems prima facie a reasonable hyJ Cereb Blood Flow Metab, Vol. 24, No. 11, 2004 pothesis because it gives reasons for the persistence of astrocyte processes around all the blood vessels—the key finding—as well as other features. On one hand, we have a role for astrocytes in integrating signals from a diversity of neurons via receptors to balance blood flow to activity (Fig. 1), and, on the other hand, we set forth that a signal of increased activity, namely glutamate, can signal the astrocyte to increase uptake of glucose from the blood and convert this to substrate for the neurons in the form of lactate in a simple servo-feedback type mechanism. However, the evidence for this is inconsistent, if not in opposition, and the data fit other scenarios as has recently been put forward in considerable detail (Chih and Roberts, 2003). The need for a hypothesis is that thinking about why we are doing particular measurements in terms of how they advance understanding is the most important aspect. Here is where the need for a hypothesis is advanced as necessary: to avoid indiscriminate collection of data with no end in mind, to force the scientist to have an end, and clarify for others what that end is. However, I hope I have pointed out that hypotheses only illustrate what one is driving toward. They do not determine that one is on the right track, and insisting upon them as a prerequisite to any proposed scientific investigation means that areas in which there is insufficient information to propose any sensible hypothesis will not be investigated or only investigated clandestinely. Looking at history, we see that it is not only not how major discoveries in the biological science have been made, but the proposal of hypotheses depends upon data in the inductive scientific method pioneered by Isaac Newton and which is the same method as we use today (Feynman, 1965). What if the existent data seems insufficient to an individual investigator to propose a sensible hypothesis? It then becomes a matter of opinion, which will be decided first by one’s peers and then by history. A not entirely satisfactory system but perhaps, like democracy, better than any others one can think of. It would be useful for the modern experimental biological sciences if this intellectual straight-jacket was recognized for what it is: simply a useful but easily disposed of intellectual technique. Then we would not get into these unending either/or arguments. The question of what mechanisms are subserved by the astrocytic processes that persist around the CNS blood vessels, and also the astrocytic processes that surround synapses (Araque et al., 1999), are critical ones that beg for answers. After sufficient data have been obtained to suggest a plausible mechanism, which as discussed above is a matter of opinion, the essence of the scientific method is that we test the implications of these mechanisms to see if they correspond with reality (Feynman, 1965). These tests have to be specific in that no reasonable COMMENTARY: THE ROLE OF HYPOTHESES IN CURRENT RESEARCH alternative mechanism should lead to the same phenomena. One must test the actual hypothesis directly: if one is talking about how astrocytes behave in the brain, the tests must be performed in the brain. Reductionism in the sense of using simpler and more experimentally tractable systems can only be suggestive and are in the final analysis not compelling. Thus after Kuffler et al. (1966) usefully used the simple leech nervous system and then the amphibian optic nerve to show that glial cells were actually high K+ cells and their membranes showed selective K+ currents, rather than high Na cells that functioned as the extracellular space of the brain (Grossman, 1972), a number of studies addressed themselves to whether the same properties translated to mammalian glia and specifically astroglia (Somjen, 1975). These investigations are still ongoing because the ion channels of astrocyte membranes are more complex than indicated in the earlier electrophysiologic experiments (Barres et al., 1990; Sontheimer, 1995; Verkhratsky and Steinhauser, 2000). REFERENCES Abbott NJ (2002) Astrocyte-endothelial interactions and blood-brain barrier permeability. J Anat 200:629–638 Allaman I, Pellerin L, Magistretti PJ (2000) Protein targeting to glycogen mRNA expression is stimulated by noradrenaline in mouse cortical astrocytes. Glia 30:382–391 Anderson CM, Nedergaard M (2003) Astrocyte-mediated control of cerebral microcirculation. Trends Neurosci 26:340–344 Araque A, Parpura V, Sanzgiri RP, Haydon PG (1999) Tripartite synapses: Glia, the unacknowledged partner. Trends Neurosci 22:208– 215. Barres BA, Chun LLY, Corey DP (1990) Ion channels in vertebrate glia. Ann Rev Neurosci 13:441–474 Chih CP, Roberts EL Jr. (2003) Energy substrates for neurons during neural activity: A critical review of the astrocyte-neuron lactate shuttle hypothesis. J Cereb Blood Flow Metab 23:1263–1281 Christianson GE (1984) In the presence of the creator: Isaac Newton and his times. New York, NY: The Free Press, MacMillan Inc. p 165 and p 188 Edvinsson L, MacKenzie ET, McCulloch J (1993) Vascular smooth muscle reactivity in vitro and in situ. In: Cerebral blood flow and metabolism, New York, NY: Raven Press, pp 113–141. Feynman R (1965) The character of physical law. Cambridge, MA: The MIT Press Golgi C (1885) Sulla fina anatomia degli organi centrali del sisterma nervoso. Riv Sper Fremiat Med Leg Alienazione Ment 11:72–123 Grossman RG (1972) The glia. In: Scientific foundations of neurology (Critchley M, O’Leary JL, Jennet B, eds), London, England: Wm. Heinemann Medical Books, Ltd, pp 9–21 Hauck SM, Suppmann S, Ueffing M (2003) Proteomic profiling of primary retinal Muller glia cells reveals a shift in expression patterns upon adaptation to in vitro conditions. Glia 44:251–263 Hoozemans JJM, Rozemuller AJM, Janssen I, De Groot CJA, Veerhuis R, Eikelenboom P (2001) Cyclooxygenase expression in microglia and neurons in Alzheimer’s disease and control brain. Acta Neuropathologica 101:2–8 Kimelberg HK (1988) Glial cell receptors. New York, NY: Raven Press. Kimelberg HK (1995) Receptors on astrocytes—what possible functions? Neurochem Intl 26:27–40 Kimelberg HK, Cai Z, Rastogi P, Charniga C, Goderie S, Dave V, Jalonen T. (1997) Transmitter-induced calcium responses differ in astrocytes acutely isolated from rat brain and in culture. J Neurochem 68:1088–1098 1239 Kuffler SW, Nicholls JG, Orkand RK (1966) Physiological properties of glial cells in the central nervous system of amphibia. J Neurophysiol 29:768–787 Leino RL, Gerhart DZ, van Bueren AM, McCall AL, Drewes LR 1997 Ultrastructural localization of GLUT 1 and GLUT 3 glucose transporters in rat brain. J Neurosci Res 49:617–626 Lindh AG (1993) Did Popper solve Hume’s problem? Nature 366:105 Magistretti PJ, Sorg O, Naichen Y, Pellerin L, De Rham S, Martin J-L (1994) Regulation of astrocyte energy metabolism by neurotransmitters. Renal Physiol Biochem 17:168–171 Mulligan SJ, MacVicar BA (2004) Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature 9;431(7005): 195–199 Nedergaard M, Ransom B, Goldman SA (2003) New roles for astrocytes: Redefining the functional architecture of the brain. Trends Neurosci 26:523–530 Newman EA (1987) Does the release of potassium from astrocyte endfeet regulate cerebral blood flow? Science 237:896–898 Pardridge WM (1997) Drug delivery to the brain. J Cereb Blood Flow Metab 17:713–731 Pellerin L, Magistretti PJ (2003) Food for thought: Challenging the dogmas. J Cereb Blood Flow Metab 23:1282–1286 Pellerin L, Pellegri G, Bittar PG, Charnay Y, Bouras C, Martin JL, Stella N, Magistretti PJ (1998) Evidence supporting the existence of an activity-dependent astrocyte-neuron lactate shuttle. DevNeurosci 20:291–299 Peng L, Arystarkhova E, Sweadner KJ (1998) Plasticity of Na,KATPase isoform expression in cultures of flat astrocytes: Species differences in gene expression. Glia 24:257–271 Porter JT, McCarthy KD (1997) Astrocytic neurotransmitter receptors in situ and in vivo. Prog Neurobiol 51:439–455 Ramón y Cajal S (1913) Contribucion al conocimento de la neuroglia del cerebro humano. Trab Lab Invest Biol Univ Madrid 11:255– 315 Sibson NR, Dhankhar A, Mason GF, Rothman DL, Beahr KL, Shulman RG (1998) Stoichiometric coupling of brain glucose metabolism and glutamatergic neuronal activity. Proc Nat Acad Sci U S A 95:316–321 Simard M, Arcuino G, Takano T, Liu QS, Nedergaard M (2003) Signaling at the gliovascular interface. J Neurosci 23:9254–9262. Somjen GG (1975) Electrophysiology of neuroglia. Ann Rev Physiol 37 163–190. Sontheimer H (1995) Ion channels in inexcitable cells. The Neuroscientist 1:64–67 Traystman RJ (1997) Regulation of cerebral blood flow by carbon dioxide. In: Primer on Cerebrovascular Disease (Welch KMA, Caplan LR, Reis DJ, Siesjo BK, Weir B, eds), San Diego, CA: Academic Press, pp 55–58 Tschirgi RD (1958) The blood-brain barrier. In: Biology of neuroglia (Windle WF, ed) Springfield, IL: Charles C. Thomas, pp 130–138 Verkhratsky A, Steinhauser C (2000) Ion channels in glial cells. Brain Res Rev 32:380–412 Virgintino D, Monaghan P, Robertson D, Errede M, Bertossi M, Ambrosi G, Roncali L (1997) An immunohistochemical and morphometric study on astrocytes and microvasculature in the human cerebral cortex. Histochem J 29:655–660 Voutsinos-Porche B, Bonvento G, Tanaka K, Steiner P, Welker E, Chatton JY, Magistretti PJ, Pellerin L (2003) Glial glutamate transporters mediate a functional metabolic crosstalk between neurons and astrocytes in the developing mouse cortex. Neuron 37:275– 286 Zhou M, Kimelberg HK (2001) Freshly isolated hippocampal CA1 astrocytes comprise two populations differing in glutamate transporter and AMPA receptor expression. J Neurosci 21:7901–7908 Zhu Y, Kimelberg HK (2004) Cellular expression of P2Y and ß-AR receptor mRNAs and proteins in freshly isolated astrocytes and tissue sections from the CA1 region of P8–12 rat hippocampus. Dev Brain Res 148:77–87 Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, Carmignoto G (2002) Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci 6:43–50 J Cereb Blood Flow Metab, Vol. 24, No. 11, 2004 1240 L. PELLERIN AND P. MAGISTRETTI Letter to the Editor Empiricism and Rationalism: Two Paths Toward the Same Goal We appreciate the scholarly contribution of Dr. Kimelberg, although we do not share his pessimistic view about hypothesis-driven research, nor do we embrace his position upon how research should be conducted. Of course, we do not believe in the supremacy of one form of scientific enquiry over the others, even when promoted by a giant such as Isaac Newton. We rather prefer the pluralistic view that there are many different ways to probe the mysteries of nature, all of them being equally valuable. Some researchers like to collect data, others prefer to formulate hypotheses and try to confirm or infirm them. Some individuals formulate theories, leaving the task to skilled experimentalists to find out whether data support proposed models. Finally, for many of us, several of these different strategies might be used at one point or another during our work. It is important to recognize that all of these approaches (or stages toward a coherent explanation of natural phenomena) play an important role in the progress of science, and even if one were to have the stature of Newton in his or her field, it appears presumptuous to recommend to future generations of scientists to favor one particular mode of investigation. The development of modern physics is there to remind us that it was fortunate that some individuals could escape the Newtonian vision of the world and formulate their own models, sometimes out of very little data, to be proven experimentally only decades later. As nicely summarized by Changeux (2004), the debate between empiricists and rationalists started by Aristotle and Plato, and then pursued by Bacon, Locke, and Hume on the empiricist side and by Descartes and Kant on the rationalist side, to name a few, is still ongoing. According to empiricists, theory should come only after the facts. The rationalist view calls for an anticipatory role of theory (models), which in this way can structure and orient experimentation. Changeux goes on to argue that the two approaches may reflect two modes of functioning of the brain, which are influenced by the sociocultural environment. In biology, studies performed upon in vitro preparations have demonstrated their capacity to provide tremendous insights about the cellular and molecular mechanisms operating in various tissue, including the central nervous system. The formulation of hypotheses based upon in vitro data represents the best way such insights can be rigorously expressed and disseminated in Received March 16, 2004; final version received June 16, 2004; accepted June 21, 2004. Address correspondence and reprint requests to Dr. Pellerin, Institut de Physiologie, 7 Rue de Bugnon, 1005 Lausanne, Switzerland; e-mail: luc.pellerin@physiol.unil.ch J Cereb Blood Flow Metab, Vol. 24, No. 11, 2004 the scientific literature to be tested by many researchers with different approaches. Of course, we agree with Dr. Kimelberg that it is important to obtain at some point a validation in vivo of hypotheses developed on the basis of in vitro studies. This is precisely what we have done for the lactate shuttle model that was elaborated and refined on the basis of a series of in vitro experiments (Bouzier-Sore et al., 2003; Brunet et al., 2004; Chatton et al., 2003; Debernardi et al., 2003; Pellerin and Magistretti, 1994, 1996, 1997; Pellerin et al., 1998a; Pierre et al., 2003), whereas some aspects were subsequently tested ex vivo and in vivo (Bittar et al., 1996; Cholet et al., 2001, 2002; Pellerin et al., 1998b; Pierre et al., 2000, 2002; Voutsinos-Porche et al., 2003). In fact, it is doubtful that in vivo experiments would have been performed without the insight provided by in vitro studies. Considering the number of interesting findings that have been made so far and the perspectives opened by the aforementioned hypothesis with new experiments being pursued by different laboratories, we can (still) predict a bright future to hypothesis-driven research. In this regard, it is instructive to consider Louis Pasteur’s statement (1880): “The illusions of the experimenter form a great part of his power. These are the preconceived ideas which serve to guide him. Many of these vanish along the path which he must travel, but one fine day he discovers and proves that some of them are adequate to the truth. Then he finds himself master of facts and new principles, the applications of which, sooner or later, bestow their benefits.” Luc Pellerin and Pierre J. Magistretti Department of Physiology Université de Lausanne Switzerland REFERENCES Bittar PG, Charnay Y, Pellerin L, Bouras C, Magistretti PJ (1996) Selective distribution of lactate dehydrogenase isoenzymes in neurons and astrocytes of human brain. J Cereb Blood Flow Metab 16:1079–1089 Bouzier-Sore AK, Voisin P, Canioni P, Magistretti PJ, Pellerin L (2003) Lactate is a preferential oxidative energy substrate over glucose for neurons in culture. J Cereb Blood Flow Metab 23: 1298–1306 Brunet JF, Grollimund L, Chatton JY, Lengacher S, Magistretti PJ, Villemure JG, Pellerin L (2004) Early acquisition of typical metabolic features upon differentiation of mouse neural stem cells into astrocytes. Glia 46:8–17 Changeux JP (2004) The physiology of truth: Neuroscience and knowledge. Cambridge, MA: Harvard University Press Chatton JY, Pellerin L, Magistretti PJ (2003) GABA uptake into astrocytes is not associated with significant metabolic cost: implications for brain imaging of inhibitory transmission. Proc Natl Acad Sci U S A 100:12456–12461 THE ASTROCYTE-NEURON LACTATE SHUTTLE Cholet N, Pellerin L, Magistretti PJ, Hamel E (2002) Similar perisynaptic glial localization for the Na+,K+-ATPase alpha 2 subunit and the glutamate transporters GLAST and GLT-1 in the rat somatosensory cortex. Cereb Cortex 12:515–525 Cholet N, Pellerin L, Welker E, Lacombe P, Seylaz J, Magistretti PJ, Bonvento G (2001) Local injection of antisense oligonucleotides targeted to the glial glutamate transporter GLAST decreases the metabolic response to somatosensory activation. J Cereb Blood Flow Metab 21:404–412 Debernardi R, Pierre K, Lengacher S, Magistretti PJ, Pellerin L (2003) Cell-specific expression pattern of monocarboxylate transporters in astrocytes and neurons observed in different mouse brain cortical cell cultures. J Neurosci Res 73:141–155 Pellerin L, Magistretti PJ (1994) Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci U S A 91:10625– 10629 Pellerin L, Magistretti PJ (1996) Excitatory amino acids stimulate aerobic glycolysis in astrocytes via an activation of the Na+/K+ ATPase. Dev Neurosci 18:336–342 Pellerin L, Magistretti PJ (1997) Glutamate uptake stimulates Na+,K+ATPase activity in astrocytes via activation of a distinct subunit highly sensitive to ouabain. J Neurochem 69:2132–2137 Pellerin L, Pellegri G, Bittar PG, Charnay Y, Bouras C, Martin JL, 1241 Stella N, Magistretti PJ (1998a) Evidence supporting the existence of an activity-dependent astrocyte-neuron lactate shuttle. Dev Neurosci 20:291–299 Pellerin L, Pellegri G, Martin JL, Magistretti PJ (1998b) Expression of monocarboxylate transporter mRNAs in mouse brain: support for a distinct role of lactate as an energy substrate for the neonatal vs. adult brain. Proc Natl Acad Sci U S A 95:3990–3995 Pierre K, Debernardi R, Magistretti PJ, Pellerin L (2003) Noradrenaline enhances monocarboxylate transporter 2 expression in cultured mouse cortical neurons via a translational regulation. J Neurochem 86:1468–1476 Pierre K, Magistretti PJ, Pellerin L (2002) MCT2 is a major neuronal monocarboxylate transporter in the adult mouse brain. J Cereb Blood Flow Metab 22:586–595 Pierre K, Pellerin L, Debernardi R, Riederer BM, Magistretti PJ (2000) Cell-specific localization of monocarboxylate transporters, MCT1 and MCT2, in the adult mouse brain revealed by double immunohistochemical labeling and confocal microscopy. Neuroscience 100:617–627 Voutsinos-Porche B, Bonvento G, Tanaka K, Steiner P, Welker E, Chatton JY, Magistretti PJ, Pellerin L (2003) Glial glutamate transporters mediate a functional metabolic crosstalk between neurons and astrocytes in the mouse developing cortex. Neuron 37:275– 286 The Astrocyte-Neuron Lactate Shuttle: A Challenge of a Challenge Leif Hertz College of Basic Medical Sciences, China Medical University, Shenyang, China; and the Department of Pharmacology, University of Saskatchewan, Saskatoon, Canada The Journal of Cerebral Blood Flow and Metabolism is to be applauded for opening up a discussion on the astrocyte-neuron lactate shuttle hypothesis (Chih and Roberts, 2003; Pellerin and Magistretti, 2003). It is also helpful to know that some of the prepositions made by Pellerin et al. (1998) and Magistretti et al. (1999) apparently have been deleted from the present version of the astrocyte-neuron lactate shuttle hypothesis (Pellerin and Magistretti, 2003), for example, that an activity-dependent aerobic glycolysis in astrocytes, triggered by glutamate uptake and glutamine synthesis, sets the pace for total energy metabolism in brain cortex (Magistretti et al., 1999). Accordingly, the present definition of the shuttle model has been modified as consisting of two, and only two, components. These are i) enhancement of aerobic glycolysis in astrocytes in response to neuronal activation of glutamatergic synapses, reflecting a dependency of astrocytic glutamate uptake upon glycolytically derived energy, and ii) oxidation in neurons of lactate produced by astrocytes. Address correspondence and reprint requests to Dr. Hertz, RR 2, Box 245, Gilmour, K0L 1W0, Ontario, Canada; e-mail: lhertz@ northcom.net Does astrocytic glutamate uptake depend upon aerobic glycolysis? The observation by Pellerin and Magistretti (1994) that uptake of glutamate by astrocytes in primary cultures causes a distinct stimulation of deoxyglucose (DG) phosphorylation and lactate production was almost immediately confirmed by Takahashi et al. (1995) in the Sokoloff laboratory, but the magnitude of the response was much smaller. However, other investigators have found that glutamate either has no effect upon DG phosphorylation and glucose use in cultured astrocytes or that it causes a decrease rather than an increase, except under anoxic conditions, suggesting that glutamate normally may be oxidized as an alternative fuel (Dienel and Cruz, 2004; Hertz et al., 1998; Liao and Chen, 2003; Peng et al., 2001; Qu et al., 2001; Swanson et al., 1990). It is known that glutamate is oxidatively degraded not only in cultured astrocytes (Hertz and Hertz, 2003; McKenna et al., 1996; Yu et al., 1982, 1992), but also in brain slices and in the brain in situ (Taylor et al., 1996; Zielke et al., 1998). Moreover, it is well established that glutamate uptake into cultured astrocytes can be fueled by either glycolytically or oxidatively derived energy (Huang et al., 1993; Swanson and Benington, 1996). J Cereb Blood Flow Metab, Vol. 24, No. 11, 2004 1242 L. HERTZ A possible explanation of the discrepancy between the results obtained by the Pellerin-Magistretti-Sokoloff groups and those reporting lack of stimulation of astrocytic glycolysis by glutamate is that the cultures used by the former groups resort to accumulating glutamate by the aid of glycolytically derived energy because they are deficient in oxidative metabolism. Many types of cultured cells, including brain cells (Dittmann et al., 1973), tend to become glycolytic and show a reduced oxidative metabolism (Guminska et al., 1969; Langvad, 1970). The predominance of glycolysis in cultured cells appears to a large extent to depend upon the culturing technique (Felder et al., 2002; Gstraunthaler et al., 1999). It can be counteracted by improved culturing conditions, especially by facilitating oxygen diffusion to the tissue (Booher et al., 1971; Kondo et al., 1997) and by not using excessive glucose concentrations (such as the 25 mM glucose used by the Pellerin-Magistretti-Sokoloff groups) in the medium (Abe et al., 2003). The Pellerin-Magistretti group has never provided any values for oxidative metabolism in their astrocyte preparations, but Itoh et al. (2003) have shown that the cultures used by the Sokoloff group have a low rate of oxidative metabolism of [U-14C]glucose (nominal rate 0.12 nmol/[min/mg protein]). This rate is five times lower than the respiratory rate observed by the same authors in cultured neurons. A five times lower respiratory rate in astrocytes than in neurons cannot reflect the in vivo situation because it has recently been demonstrated by three groups that astrocytes, which occupy less than 30% of the volume in the brain cortex (Pope, 1978; Williams et al., 1980; Wolff and Chao, 2004), account for approximately 15% of its oxidative metabolism (Blüml et al., 2002; Gruetter et al., 2001; Lebon et al., 2002). Also, substantially higher respiratory rates (approximately 1.0 nmol/[min/mg protein]) have been reported by Hertz and coworkers in both astrocytes and cerebellar granule neurons with [U-14C]glucose as the precursor (e.g., Peng et al., 1994; Yu and Hertz, 1983) and by Lopes-Cardozo et al. (1986), using [2-14C]glucose, and Vicario et al. (1993) found virtually identical respiratory rates in cultures of neurons and astrocytes. Accordingly, it cannot be concluded that cultured astrocytes depend upon glycolysis for glutamate uptake. However, this does not negate the possibility that there could be situations in the brain in vivo, where glutamate uptake is fueled by glycolytically derived energy (e.g., in the most peripheral parts of both neurons and astrocytes, which are too minute to contain mitochondria). It has been used as a powerful argument in favor of the astrocyte-neuron lactate shuttle that interference with astrocytic glutamate uptake decreases glucose utilization during in vivo activation of the brain, including its barrel cortex, regardless whether the inhibition occurs by administration of the transport inhibitors beta-D,LJ Cereb Blood Flow Metab, Vol. 24, No. 11, 2004 threohydroxyaspartate (THA) or pirrolidine-2-4dicarboxylate (PDC) (Demestre et al., 1997), by injection of antisense mRNA to the transporter (Cholet et al., 2001) or by the use of mutant mice without one or the other of the two astrocytic glutamate transporters GLT-1 and GLAST (Voutsinos-Porche et al., 2003). However, these observations are not conclusive. One problem is that 10-day-old rats were used for these experiments. Oxidative metabolism of glucose is not fully developed in the 10-day-old rat or mouse brain. Ketone bodies, which use the monocarboxylic acid transporter (MCT) for uptake, are an important fuel for suckling rats (Cremer, 1982; Medina et al., 1999; Nehlig, 1999), and the rate of glutamate oxidation in cultured mouse astrocytes is lower in cells corresponding to postnatal day 10 than in more mature cells (Yager et al., 1994). It would, therefore, have been preferable to use adult animals in the study by Voutsinos-Porche et al. (2003), especially since the authors indicate that the mutant mice may mature at a reduced rate. Another caveat against the conclusion that the reduced metabolic response to brain activation must be a reflection of a diminished workload by the astrocytes is provided by a multitude of experimental observations that many facets of glutamatergic activities are impaired when glutamate uptake is reduced (e.g., Maki et al., 1994; Niederberger et al., 2003; Turecek and Trussel, 2000). Inhibition of glutamate uptake might lead to reduction of presynaptic glutamate release, activation of inhibitory metabotropic glutamate receptors, postsynaptic desensitization, or disturbance of glutamate recycling. Excitatory postsynaptic currents in the rat barrel cortex are inhibited by glutamate uptake inhibitors, and neurons in the developing neocortex are particularly sensitive to glutamate transporter function (Kidd and Isaac, 2000). As indicated in the report by Voutsinos-Porche et al. (2003), several aspects of glutamatergic transmission are unaltered in the adult mutant mice used in their work. However, others are not. In the very same GLT-1−/− mutant as that used by Voutsinos-Porche et al. (2003), long-term potentiation (LTP) induced by tetanic stimulation is reduced by two-thirds (Katagiri et al., 2001). Development of LTP in brain slices is known to enhance Na+,K+-ATPase activity (Glushchenko and Izvarina, 1997) and thus increases energy demand. It must therefore be concluded that neither studies in cultured astrocytes nor in vivo studies in animals with glutamate transporter deficiencies prove the dependence of astrocytic glutamate transport upon aerobic glycolysis. How much lactate is produced in activated brain in vivo, and is there a substantial lactate flux through extracellular fluid? An essential component of the lactate shuttle hypothesis is that there must be a large, directed transfer of THE ASTROCYTE-NEURON LACTATE SHUTTLE lactate from astrocytes to neurons in vivo, but this has never been demonstrated. It is well established that in spite of sufficient oxygen availability, activation of brain leads to an increase in intracellular and extracellular lactate from a “resting” value of approximately 1.0 mol/g wet wt. and 1 mM, respectively, to approximately twice these values, and that lactate rapidly returns to its previous level after the activation is terminated (Dienel et al., 2002; Korf; 1996; Prichard et al., 1991). However, it is uncertain whether this lactate simply represents a larger, static pool, that is, an increased level of tissue lactate arising from the need of a higher concentration of pyruvate to stimulate pyruvate dehydrogenase activity sufficiently for TCA cycle flux to match an increased rate of glycolysis. To demonstrate a large flux from astrocytes to neurons through the enlarged lactate pool requires determination of the cells that are the main lactate producers and the main lactate consumers in the brain as well as quantification of lactate production and degradation. Which cell type is the predominant producer of lactate in the brain in vivo? It has not been established with certainty from which cell type(s) lactate is released during brain activation in vivo nor by which mechanism(s) the resting level is reestablished. There is evidence that at least some of the released lactate originates from astrocytes. Thus microdialysis performed 1 to 2 weeks after the insertion of a microdialysis probe, when the probe is surrounded by an adhering scar of reactive glia, shows a stress-induced increase in extracellular lactate, which is similar to that seen 1 to 2 days after the insertion of the probe, when access of neuronally released compounds to the probe is indicated by a large K+-mediated transmitter release (Korf, 1996). This observation does not, however, exclude the possibility that neurons also release lactate. Because stimulation of glucose use in response to increased energy demand is fundamental to regulation of cellular energy metabolism (see, e.g., Chih and Roberts, 2003; Hertz and Dienel, 2002), and because neurons are well equipped with glucose transporters (Dwyer et al., 2002), it is difficult to envision that glycolysis in neurons should be unaffected when neuronal energy requirements are increased. Moreover, the glycolytic enzyme hexokinase has a high activity in synaptosomes (Wilson, 1972), glycolytic ATP formation has been demonstrated in postsynaptic densities (Wu et al., 1997), and in cultured glutamatergic neurons the rate of 14CO2 production from labeled glucose is as high as in astrocytes and distinctly increased during K+-mediated depolarization (Peng et al., 1994). In cell cultures, both neurons and astrocytes release large amounts of lactate to the medium, although the release from astrocytes (25–35 nmol/min/mg protein) is 1243 two to three times larger than the neuronal release (5–11 nmol/min/mg protein) (Dienel and Hertz, 2001; Schousboe et al., 1997; Waagepetersen et al., 2000; Walz and Mukerji, 1988). Monocarboxylate transporter (MCT)mediated facilitated diffusion, which is driven by concentration gradients, is responsible for lactate transport in and out of cells (Halestrap and Price, 1999; Juel, 2001), and a major reason for the high rates of membrane transport in the cultured cells is the large volume of extracellular medium, which initially contains no lactate. Because of the slow rise of the extracellular lactate concentration, a concentration gradient can be maintained between even a low intracellular lactate concentration and the extracellular lactate concentration, promoting continuous release of lactate. In the brain in vivo lactate release would be delayed by rapid increase in adjacent extracellular fluid. The rate of continued release of lactate would be determined by its removal by extracellular diffusion, uptake into adjacent cells with a lower concentration of lactate, and under a few pathologic conditions (Dienel and Cruz, 2003) lactate release to the circulation. Lactate production in astrocytes occurs not only during the glycolytic part of glucose degradation, but also during breakdown of glycogen (Dringen et al., 1993), which is stimulated during brain activation and can be very fast (Dienel et al., 2002; Swanson, 1992; Swanson et al., 1992). Because glycogen and activity of its degrading enzyme, glycogen phosphorylase, within brain parenchyma is virtually restricted to astrocytes (Ibrahim, 1975; Richter et al., 1996), its breakdown products must be released from astrocytes or further metabolized in astrocytes. The content of glycogen in brain, and thus also activity-induced glycogenolysis, is larger than previously recognized (Cruz and Dienel, 2002; Kong et al., 2002), and during brain activation the rate of lactate equivalents produced from glycogen exceeds that of lactate production from glucose (Dienel and Cruz, 2003, 2004). Within the activated tissue formation of lactate from glycogen, which under generally used experimental conditions is unlabeled, should therefore be expected to greatly dilute the specific activity of lactate generated from [14C]labeled glucose. However, recent experiments have shown that the specific activity of lactate produced in the brain from the administered labeled glucose is not diluted by any nonlabeled lactate (Dienel et al., 2002). This remarkable observation demonstrates that the two pools of lactate (and pyruvate), one from glycogen and one from the [14C]glucose delivered by blood, are segregated. A segregation can be explained by cytosolic “channeling” of metabolites from one enzyme to the next in a pathway. Such a channeling is known in vascular smooth muscle, where lactate formed by glycolysis is segregated from that formed by glycogenolysis (Allen J Cereb Blood Flow Metab, Vol. 24, No. 11, 2004 1244 L. HERTZ and Hardin, 2000; Lynch and Paul, 1983). The channeling may not be restricted to the individual astrocyte in which lactate was produced. Rather, glycogen-derived lactate appears to be transported away from the activated area, probably by gap-junction mediated transport to other cells in the astrocytic syncytium (Medina et al., 1999). A segregation of lactate obtained by glycolysis and glycogenolysis is virtually incompatible with release of astrocytic lactate from both glucose and glycogen to a presumably nonchanneled extracellular space (a precondition for an astrocyte-neuron lactate shuttle). Does oxidation of lactate primarily occur in neurons? The concept that glucose-derived lactate, released from astrocytes, is a preferred substrate for neurons is a key component of the shuttle model. It has been taken as support of this hypothesis that Itoh et al. (2003) and Bouzier-Sore et al. (2003, 2004) found that unlabeled lactate profoundly reduced the production of 14CO2 from labeled glucose in hippocampal and cortical neurons, respectively. In contrast, there was little, if any, inhibition by unlabeled glucose of production of 14CO2 from labeled lactate. The main reason for the inhibition of neuronal 14CO2 production from labeled glucose by unlabeled lactate was postulated by Itoh et al. (2003) to be that conversion of lactate to pyruvate converts NAD+ to NADH + H+ and therefore reduces the availability of NAD+ for oxidation of glyceraldehyde-3-phosphate during glycolysis. However, if this mechanism were the major reason for the reduced 14CO2 production from [14C]glucose in the presence of lactate, one would expect that pyruvate, which does not consume NAD+ before its oxidation, would be a less efficient inhibitor of 14CO2 production from [14C]glucose than lactate. In fact, the opposite is the case, because unpublished experiments by Peng and Hertz have shown that 14CO2 production from 7.5 mM [14C]glucose in the glutamatergic cerebellar granule neurons is reduced from 1.3 ± 0.19 to 0.29 ± 0.02 nmol/min/mg protein (n ⳱ 6), that is, by 77.1%, in the presence of 5 mM pyruvate, but only by 47.1% in the presence of 5 mM lactate (pyruvate vs. lactate: P < 0.01). Larger inhibition of release of labeled CO2 by addition of unlabeled pyruvate than by addition of unlabeled lactate suggests that the dilution of the specific activity of [14C]glucose-derived pyruvate/lactate by extracellular lactate or pyruvate has a greater impact than NAD+ availability. The strong influence of exogenous substrates on dilution of specific activity in endogenous pyruvate is to be expected because brain pyruvate levels are low, that is, 50 to 100 M (Siesjö, 1978). Flooding of cultured cells with large amounts of unlabeled lactate, facilitated by the high MCT activities, and a rapid and reversible interchange between lactate and pyruvate (Wolfe, 1990; Wolfe et al., 1988), overwhelm the intracellular meJ Cereb Blood Flow Metab, Vol. 24, No. 11, 2004 tabolite levels. Isotope dilution of glucose-derived pyruvate/lactate will cause a large reduction of the production of labeled CO2 from glucose, regardless of whether glucose metabolism is suppressed. That a potential impairment of glucose metabolism by lactate caused by competition for NAD+ plays at most a minor role is also suggested by the observation by Bliss and Sapolsky (2001) that DG phosphorylation in hippocampal neurons is not inhibited at physiologically relevant lactate levels and that even highly abnormal lactate/glucose ratios (e.g., 0.5 mM glucose and 5 mM lactate) have only a very modest inhibitory effect (approximately 15%). The possibility of inhibition of neuronal and astrocytic lactate oxidation by glucose has been examined experimentally by using the reverse experimental situation, that is, measurement of production of 1 4 CO 2 from [U-14C]lactate in the presence of different concentrations of glucose. Because glucose use is governed by product inhibition of key enzymes, especially phosphofructokinase (reviewed by Hertz and Dienel, 2002), little effect of elevated glucose level on lactate metabolism would be expected as long as the extracellular glucose concentration is maintained above the level necessary to secure sufficient glucose transport into the cell. This extracellular glucose level could be 2.5 mM or perhaps slightly higher because the Km for the neuronal glucose transporter is 2 to 3 mM (Maher et al., 1996). Raising the concentration of glucose above this level would not increase delivery of carbon to downstream metabolic pools, which is consistent with the observation that hyperglycemia does not change glucose use rates in brain in vivo (Orzi et al., 1988). This reasoning explains a modest effect of unlabeled glucose, regardless of its concentration, upon 14CO2 production from 2 mM [14C]lactate in the experiments by Itoh et al. (2003). The larger inhibition of 14CO2 production from [U-14C]lactate by glucose in astrocytes observed by these authors is to be expected because of the higher rate of glycolysis in the astrocytes, leading to a higher concentration of unlabeled glucosederived pyruvate/lactate, which accordingly can compete more efficiently with exogenous lactate. In two studies by Bouzier-Sore et al. (2003, 2004), a dilution of specific activity of glucose-derived pyruvate by addition of nonlabeled lactate must in a similar manner invalidate an otherwise ingenious approach to quantitate metabolism of glucose, exogenous lactate and glucosederived lactate. It is also against the notion that lactate predominantly should be oxidized in neurons that Peng et al. (1994) observed similar rates of 14CO2 formation from [14C]lactate (5 mM) in cultured GABAergic cerebral cortical neurons, cerebellar granule neurons, and astrocytes. However, using “trapping” of label from either [13C]glucose or [13C]lactate into glutamate as a determination of THE ASTROCYTE-NEURON LACTATE SHUTTLE tricarboxylic acid cycle activity, Waagepetersen et al. (1998) found that incorporation of label from [13C]lactate into glutamate in cultured cortical astrocytes was only 50% of that in cortical neurons. However, these experiments were performed with either 0.5 mM glucose or 1 mM lactate as the metabolic substrate, and stimulation of glycogenolysis in the aglycemic lactate medium may have caused a decrease in the specific activity of intracellular lactate. This type of experiment does accordingly also not provide solid evidence that lactate should be a preferred substrate for neurons. Publications by Bouzier et al. (2000) and by Tyson et al. (2003) claim to show that lactate is primarily oxidized in neurons in vivo, based upon the finding that intravenous injection of [3-13C]lactate gives rise to identical incorporation of label into the C-2 and the C-3 position of glutamate and glutamine, rather than to selective incorporation into the C-2 position, indicative of pyruvate carboxylation. Based upon this observation, the authors conclude that lactate is metabolized in a compartment expressing no pyruvate carboxylase activity, that is, a neuronal, not an astrocytic compartment. However, the oxaloacetate produced by pyruvate carboxylation is known to readily equilibrate with the symmetrical fumarate, thereby giving rise to equal labeling of C-2 and C-3 in glutamate and glutamine. For example, previous studies from the same laboratory by Merle et al. (1996) concluded, based upon a mathematical modeling of [1-13C]glucose metabolism in cultured astrocytes that 39% of oxaloacetate synthesized by pyruvate carboxylation equilibrates with fumarate before it is condensed with acetyl coenzyme A to form citrate, from which glutamate is produced. Sonnewald et al. (1993) found an even more extensive, and possibly quantitative equilibration between oxaloacetate and fumarate. Moreover, pyruvate carboxylation from exogenous lactate in intact brain has been demonstrated by other authors (Qu et al., 2000), although it was less pronounced than when glucose was the substrate. Finally, demonstration of pyruvate carboxylation provides only a positive identification of one part of astrocytic metabolism because dehydrogenation via acetyl coenzyme A in the astrocytic compartment is not determined. In fact, in intact brain the calculated rate of pyruvate dehydrogenation in astrocytes exceeds that of pyruvate carboxylation by a factor of almost 2 (Gruetter et al., 2001). Another observation in intact nervous tissue used to support the astrocyte-neuron lactate shuttle is that Schwann cells in a stimulated isolated vagus nerve preparation account for as much as 78% of the total deoxyglucose phosphorylation in the nerve (Vega et al., 2003). Pellerin and Magistretti (2003) compared this value with a calculated estimate by Attwell and Laughlin (2001) of glial expenditure of energy in cortical gray matter as 5% of total energy consumption (a value that 1245 must be too low, considering that astrocytes account for 15% of the oxygen consumption in brain), and they concluded that the most likely explanation for this apparent paradox is that the remaining 73% of phosphorylated glucose were transferred to the axon in the form of lactate. However, such a comparison between a peripheral nerve and cortical gray matter is not legitimate because experiments measuring rates of oxygen consumption in crayfish giant axons and in their ensheathing glial cells have led to the estimate that in a normally functioning axon-glial cell system the glial sheath accounts for 90% of the oxygen consumption by the tissue (Hargittai and Lieberman, 1991). Provided this value also applies to mammalian peripheral nerves, the Schwann cells themselves are likely to oxidize all glucose they phosphorylate. That mammalian Schwann cells probably have a high rate of oxidative metabolism is indicated by an extremely high mitochondrial density in these cells (Rydmark et al., 1998). Moreover, an inhibitor of the MCT supposed to carry lactate from the Schwann cells to the axon had no effect upon the action potential or the distribution of radioactivity after exposure to labeled deoxyglucose in the experiments by Vega et al. (2003). Along similar lines, mobilization of the glycogen contained in a rat optic nerve sustains axonal action potential in the absence of glucose (Wender et al., 2000). It was concluded that this may be due to lactate transport from glial cells to neurons (Brown et al., 2003), although approximately 80% of the released K+ is likely to be initially accumulated by the glial cells, mainly by active transport mechanisms (Ransom et al., 2000). Because of the narrowness of the periaxonal space and the low resting extracellular K+ concentration, the relative change in extracellular K+ during the action potential is much larger than the changes in intraaxonal ion concentrations. That accumulation of extracellular K+ during energy failure leads to axonal block long before changes in intraaxonal ion concentrations affect excitability was demonstrated by Shanes (1951), who showed that axonal conduction block in squid nerve during anoxia could be reversed by simple wash with anoxic artificial sea water. The distribution of different isotypes of MCT’s between astrocytes and neurons is also cited as being in favor of directed transport of lactate from astrocyte to neuron (Pellerin, 2003). However, because lactate transport across the cell membrane occurs by facilitated diffusion, its direction is determined by concentration gradients (not only of lactate, but also of H+, which is cotransported), not by transporter type (Halestrap and Price, 1999; Juel, 2001). Moreover, a Km value of the mainly neuronal MCT-2 for lactate is approximately 0.7 mM, whereas those of the mainly astrocytic MTC-1 and MCT-4 are 3 to 5 mM or higher (Bergersen et al., 2001; Broer et al., 1997, 1999; Dimmer et al., 2000; Halestrap J Cereb Blood Flow Metab, Vol. 24, No. 11, 2004 1246 L. HERTZ and Price, 1999); this means that they are more responsive to the increase in the lactate concentration occurring during brain activity (Hertz and Dienel, 2004). What conclusions, if any, can be made regarding a lactate shuttle? The purpose of this challenge of a challenge has been to critically evaluate the prepositions of the present version of the astrocyte-neuron lactate shuttle hypothesis: i) pronounced aerobic glycolysis, especially in astrocytes, ii) stimulation of aerobic glycolysis in astrocytes triggered by glutamate uptake, and iii) oxidation of lactate primarily in neurons. There is evidence that glycolysis combined with glycogenolysis may be more pronounced in astrocytes than in neurons, especially during brain activation. Nevertheless, neurons almost certainly carry out glycolysis and they are therefore likely to contribute substantially to aerobic glycolysis and lactate production in the brain in vivo. Glutamate uptake in astrocytes can be metabolically fueled by either glycolytically or oxidatively derived energy, but there is no good evidence that it relies upon glycolytically derived energy. Because there is net production of lactate in vivo, and in most cases no release to the circulation, it is likely that lactate is also degraded in the brain, although this process may be temporally dissociated from its production, as suggested by a mismatch between the increase in glucose use and in oxygen consumption during many types of brain activation (Fox and Raichle, 1986; Fox et al., 1988). The site of lactate use is also likely to be spatially separated from its site of production. There is, however, no solid indication that lactate oxidation should primarily occur in neurons, and there is very good evidence that it is not exclusively a neuronal process. The magnitude and direction(s) of any lactate flux(es) in the functioning brain constitute two major unknowns. If the magnitude is small, then the proposed shuttle process is of little functional significance. If it is large, then its direction(s) would be of major interest, but it could at least equally well occur within a glial syncytium as from astrocytes to neurons. REFERENCES Abe T, Takahashi S, Fukuuchi Y (2003) Limited glucose availability enhances oxidative metabolism of glucose in cultured rat astroglia. Abstracts Soc Neurosci Program number 765.4 Allen TJ, Hardin CD (2000) Influence of glycogen storage on vascular smooth muscle metabolism. Am J Physiol Heart Circ Physiol 278:H1993–H2002 Attwell D, Laughlin SB (2001) An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab 21:1133–1145 Bergersen L, Waerhaug O, Helm J, Thomas M, Laake P, Davies AJ, Wilson MC, Halestrap AP, Ottersen OP (2001) A novel postsynaptic density protein: The monocarboxylate transporter MCT2 is co-localized with delta-glutamate receptors in postsynaptic densities of parallel fiber-Purkinje cell synapses. Exp Brain Res 136: 523–534 J Cereb Blood Flow Metab, Vol. 24, No. 11, 2004 Bliss TM, Sapolsky RM (2001) Interactions among glucose, lactate and adenosine regulate energy substrate utilization in hippocampal cultures. Brain Res 899:134–141 Blüml S, Moreno-Torres A, Shic F, Nguy CH, Ross BD (2002) Tricarboxylic acid cycle of glia in the in vivo human brain. NMR Biomed 15:1–5 Booher J, Fosmark H, Hertz L (1971) Rates of oxygen uptake by dorsal root neurons cultivated in the Rose chamber in well oxygenated medium or in relative hypoxia. Neurobiology 1:32–36 Bouzier AK, Thiaudiere E, Biran M, Rouland R, Canioni P, Merle (2000) The metabolism of [3–13C]lactate in the rat brain is specific of a pyruvate carboxylase-deprived compartment. J Neurochem 75:480–486 Bouzier-Sore AK, Voisin P, Bouchaud V, Franconi J-M, Magistretti PJ, Pellerin L (2004) Contribution of lactate and glucose to neuronal oxidative metabolism in vitro. Abstracts, Sixth International Meeting for Brain Energy Metabolism, Heraklion, Greece, p 40 Bouzier-Sore AK, Voisin P, Canioni P, Magistretti PJ, Pellerin L (2003) Lactate is a preferential oxidative energy substrate over glucose for neurons in culture. J Cereb Blood Flow Metab 23: 1298–1306 Broer S, Broer A, Schneider HP, Stegen C, Halestrap AP, Deitmer JW (1999) Characterization of the high-affinity monocarboxylate transporter MCT2 in Xenopus laevis oocytes. Biochem J 341:529– 535 Broer S, Rahman B, Pellegri G, Pellerin L, Martin JL, Verleysdonk S, Hamprecht B, Magistretti PJ (1997) Comparison of lactate transport in astroglial cells and monocarboxylate transporter 1 (MCT 1) expressing Xenopus laevis oocytes. Expression of two different monocarboxylate transporters in astroglial cells and neurons. J Biol Chem 272:30096–30102 Brown AM, Tekkok SB, Ransom BR (2003) Glycogen regulation and functional role in mouse white matter. J Physiol 549:501–512 Chih C-P, Roberts EL Jr. (2003) Energy substrates for neurons during neural activity: A critical review of the astrocyte-neuron lactate shuttle hypothesis. J Cereb Blood Flow Metab 23:1263–1281 Cholet N, Pellerin L, Welker E, Lacombe P, Seylaz J, Magistretti P, Bonvento G (2001) Local injection of antisense oligonucleotides targeted to the glial glutamate transporter GLAST decreases the metabolic response to somatosensory activation. J Cereb Blood Flow Metab 21:404–412 Cremer JE (1982) Substrate utilization and brain development. J Cereb Blood Flow Metab 2:394–407 Cruz NF, Dienel GA (2002) High brain glycogen levels in brains of rats with minimal environmental stimuli: Implications for metabolic contributions of working astrocytes. J Cereb Blood Flow Metab 22:1476–1489 Demestre M, Boutelle M, Fillenz M (1997) Stimulated release of lactate in freely moving rats is dependent on the uptake of glutamate. J Physiol 499:825–832 Dienel GA, Cruz NF (2003) Neighborly interactions of metabolicallyactivated astrocytes in vivo. Neurochem Int 43:339–354 Dienel GA, Cruz NF (2004) Nutrition during brain activation: Does cell-to-cell lactate shuttling contribute significantly to sweet and sour food for thought? Neurochem Int 45:321–351 Dienel GA, Hertz L (2001) Glucose and lactate metabolism during brain activation. J Neurosci Res 66:824–838. Dienel GA, Wang RY, Cruz NF (2002) Generalized sensory stimulation of conscious rats increases labeling of oxidative pathways of glucose metabolism when the brain glucose-oxygen uptake ratio rises. J Cereb Blood Flow Metab 22:1490–1502 Dimmer KS, Friedrich B, Lang F, Deitmer JW, Broer S (2000) The low-affinity monocarboxylate transporter MCT4 is adapted to the export of lactate in highly glycolytic cells. Biochem J 350:219–227 Dittmann L, Hertz L, Fosmark H, Sensenbrenner M, Mandel P (1973) Development of respiration in fresh and cultivated brain cortex cells. Trans Biochem Soc 1:136–138 Dringen R, Gebhardt R, Hamprecht B (1993) Glycogen in astrocytes: Possible function as lactate supply for neighboring cells. Brain Res 623:208–214 Dwyer DS, Vannucci SJ, Simpson IA (2002) Expression, regulation, and functional role of glucose transporters (GLUTs) in brain. Int Rev Neurobiol 51:159–188 THE ASTROCYTE-NEURON LACTATE SHUTTLE Felder E, Jennings P, Seppi T, Pfaller W (2002) LLC-PK(1) cells maintained in a new perfusion cell culture system exhibit an improved oxidative metabolism. Cell Physiol Biochem 12:153–62 Fox PT, Raichle ME (1986) Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. Proc Natl Acad Sci U S A 83:1140–1144 Fox PT, Raichle ME, Mintun MA, Dence C (1988). Nonoxidative glucose consumption during focal physiologic neural activation. Science 241:462–464. Glushchenko TS, Izvarina NL (1997) Na+,K+-ATPase activity in neurons and glial cells of the olfactory cortex of the rat brain during the development of long-term potentiation. Neurosci Behav Physiol 27:49–52 Gruetter R, Seaquist ER, Ugurbil K (2001) A mathematical model of compartmentalized neurotransmitter metabolism in the human brain. Am J Physiol Endocrinol Metab 281:E100–E112 Gstraunthaler G, Seppi T, Pfaller W (1999) Impact of culture conditions, culture media volumes, and glucose content on metabolic properties of renal epithelial cell cultures. Are renal cells in tissue culture hypoxic? Cell Physiol Biochem 9:150–172 Guminska M, Briand P, Daehnfeldt JL, Kieler J (1969) Changes in the pyruvate kinase and lactate dehydrogenase activity in rat lung during cultivation and “spontaneous” development of malignancy in vitro. Eur J Cancer 5:597–604 Halestrap AP, Price NT (1999) The proton-linked monocarboxylate transporter (MCT) family: Structure, function and regulation. Biochem J 343:281–299 Hargittai PT, Lieberman EM (1991) Axon-glia interactions in the crayfish: Glial cell oxygen consumption is tightly coupled to axon metabolism. Glia 4:417–423 Hertz L, Dienel GA (2002) Energy metabolism in the brain. Int Rev Neurobiol 51:1–102 Hertz L, Dienel GA (2004) Lactate transport and transporters: General principles and functional roles in brain cells. J Neurosci Res, in press. Hertz L, Hertz E (2003) Determination of rate of glutamate-sustained oxygen consumption in primary cultures of astrocytes as a means to estimate cataplerotic TCA cycle flux. Neurochem Int 43:355– 361 Hertz L, Swanson RA, Newman GC, Marrif H, Juurlink BH, Peng L (1998) Can experimental conditions explain the discrepancy over glutamate stimulation of aerobic glycolysis? Dev Neurosci 20:339–347 Huang R, Shuaib A, Hertz, L (1993) Glutamate uptake and glutamate content in primary cultures of mouse astrocytes during anoxia, substrate deprivation and simulated ischemia under normothermic and hypothermic conditions. Brain Res 618:643–351 Ibrahim MZ (1975) Glycogen and its related enzymes of metabolism in the central nervous system. Adv Anat Embryol Cell Biol 52:3–89 Itoh Y, Esaki T, Shimoji K, Cook M, Law MJ, Kaufman E, Sokoloff L (2003) Dichloroacetate effects on glucose and lactate oxidation by neurons and astroglia in vitro and on glucose utilization by brain in vivo. Proc Natl Acad Sci U S A 100:4879–4884 Juel C (2001) Current aspects of lactate exchange: Lactate/H+ transport in human skeletal muscle. Eur J Appl Physiol 86:12–16 Katagiri H, Tanaka K, Manabe T (2001) Requirement of appropriate glutamate concentrations in the synaptic cleft for hippocampal LTP induction. Eur J Neurosci 14:547–553 Kidd FL, Isaac JT (2000) Glutamate transport blockade has a differential effect on AMPA and NMDA receptor-mediated synaptic transmission in the developing barrel cortex. Neuropharmacology 39:725–732 Kondo M, Tamaoki J, Sakai A, Kameyama S, Kanoh S, Konno K (1997) Increased oxidative metabolism in cow tracheal epithelial cells cultured at air-liquid interface. Am J Respir Cell Mol Biol 16:62–68 Kong J, Shepel PN, Holden CP, Mackiewicz M, Pack AI, Geiger JD, 2002. Brain glycogen decreases with increased periods of wakefulness: implications for homeostatic drive to sleep. J Neurosci 22:5581–5587 Korf J (1996) Intracerebral trafficking of lactate in vivo during stress, exercise, electroconvulsive shock and ischemia as studied with microdialysis. Dev Neurosci 18:405–414 1247 Langvad E (1970) The application of a tidal flow culture method to the study of histological and lactate dehydrogenase (LDH)-isoenzymatic changes in primary mouse lung cell cultures. Acta Pathol Microbiol Scand 78:497–504 Lebon V, Petersen KF, Cline GW, Shen J, Mason GF, Dufour S, Behar KL, Shulman GI, Rothman DL (2002) Astroglial contribution to brain energy metabolism in humans revealed by 13C nuclear magnetic resonance spectroscopy: Elucidation of the dominant pathway for neurotransmitter glutamate repletion and measurement of astrocytic oxidative metabolism. J Neurosci 22:1523–1531 Liao S, Chen CJ (2003) L-glutamate decreases glucose utilization by rat cortical astrocytes. Neurosci Lett 348:81–84 Lopes-Cardozo M, Larsson OM, Schousboe A (1986) Acetoacetate and glucose as lipid precursors and energy substrates in primary cultures of astrocytes and neurons from mouse cerebral cortex. J Neurochem 46:773–778 Lynch RM, Paul RJ (1983) Compartmentation of glycolytic and glycogenolytic metabolism in vascular smooth muscle. Science 222:1344–1346 Magistretti PJ, Pellerin L, Rothman DL, Shulman RG (1999) Energy on demand. Science 283:496–497 Maher F, Davies-Hill TM, Simpson IA (1996) Substrate specificity and kinetic parameters of GLUT3 in rat cerebellar granule neurons. Biochem J 315:827–831 Maki R, Robinson MB, Dichter MA (1994) The glutamate uptake inhibitor L-trans-pyrrolidine-2,4-dicarboxylate depresses excitatory synaptic transmission via a presynaptic mechanism in cultured hippocampal neurons. J Neurosci 14:6754–6762 McKenna MC, Sonnewald U, Huang X, Stevenson J, Zielke HR (1996) Exogenous glutamate concentration regulates the metabolic fate of glutamate in astrocytes. J Neurochem 66:386–393 Medina JM, Giaume C, Tabernero A (1999) Metabolic coupling and the role played by astrocytes in energy distribution and homeostasis. Adv Exp Med Biol 468:361–371 Merle M, Martin M, Villegier A, Canioni P (1996) Mathematical modeling of the citric acid cycle for the analysis of glutamine isotopomers from cerebellar astrocytes incubated with [1-13C]glucose. Eur J Biochem 239:742–751 Nehlig A (1999) Age-dependent pathways of brain energy metabolism: The suckling rat, a natural model of the ketogenic diet. Epilepsy Res 37:211–221 Niederberger E, Schmidtko A, Rothstein JD, Geisslinger G, Tegeder I (2003) Modulation of spinal nociceptive processing through the glutamate transporter GLT-1. Neuroscience 116:81–87 Orzi F, Lucignani G, Dow-Edwards D, Namba H, Nehlig A, Patlak CS, Pettigrew K, Schuier F, Sokoloff L (1988) Local cerebral glucose utilization in controlled graded levels of hyperglycemia in the conscious rat. J Cereb Blood Flow Metab 8:346–356 Pellerin L (2003) Lactate as a pivotal element in neuron-glia metabolic cooperation. Neurochem Int 43:331–338 Pellerin L, Magistretti PJ (1994) Glutamate uptake into astrocytes stimulates aerobic glycolysis: A mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci U. S. A. 91:10625– 10629 Pellerin L, Magistretti PJ (2003) Food for thought: Challenging the dogmas. J Cereb Blood Flow Metab 23:1282–1286 Pellerin L, Pellegri D, Bittar PG, Charnay Y, Bouras C, Martin J-L, Stella N, Magistretti PJ (1998) Evidence supporting the existence of an activity-dependent astrocyte-neuron lactate shuttle. Dev Neurosci 20:291–299 Peng L, Zhang X, Hertz L (1994) High extracellular potassium concentrations stimulate oxidative metabolism in a glutamatergic neuronal culture and glycolysis in cultured astrocytes but have no stimulatory effect in a GABAergic neuronal culture. Brain Res 663:168–172 Peng L, Swanson RA, Hertz L (2001) Effects of L-glutamate, Daspartate, and monensin on glycolytic and oxidative glucose metabolism in mouse astrocyte cultures: further evidence that glutamate uptake is metabolically driven by oxidative metabolism. Neurochem Int 38:437–443 Pope A (1978) Neuroglia: quantitative aspects. In: Dynamic Properties of Glial Cells (Schoffeniels E, Franck G, Hertz L, Tower DB, eds), Oxford: Pergamon Press, pp 13–20 J Cereb Blood Flow Metab, Vol. 24, No. 11, 2004 1248 L. HERTZ Prichard J, Rothman D, Novotny E, Petroff O, Kuwabara T, Avison M, Howseman A, Hanstock C, Shulman R (1991) Lactate rise detected by 1H NMR in human visual cortex during physiologic stimulation. Proc Natl Acad Sci U S A 88:5829–5831 Qu H, Håberg A, Haraldseth O, Unsgård G, Sonnewald U (2000) 13C MR spectroscopy study of lactate as substrate for rat brain. Dev Neurosci 22:429–436 Qu H, Eloqayli H, Unsgård G, Sonnewald U (2001) Glutamate decreases pyruvate carboxylase activity and spares glucose as energy substrate in cultured cerebellar astrocytes. J Neurosci Res 66: 1127–1132 Ransom CB, Ransom BR, Sontheimer H (2000) Activity-dependent extracellular K+ accumulation in rat optic nerve: The role of glial and axonal Na+ pumps. J Physiol 522:427–42 Richter K, Hamprecht B, Scheich H (1996) Ultrastructural localization of glycogen phosphorylase predominantly in astrocytes of the gerbil brain. Glia 17:263–273 Rydmark M, Berthold CH, Gatzinsky KP (1998) Paranodal Schwann cell mitochondria in spinal roots of the cat. An ultrastructural morphometric analysis. J Neurocytol 27:99–108 Schousboe A, Westergaard N, Waagepetersen HS, Larsson OM, Bakken IJ, Sonnewald U (1997) Trafficking between glia and neurons of TCA cycle intermediates and related metabolites. Glia 21:99– 105 Shanes AM (1951) Potassium movement in relation to nerve activity. J Gen Physiol 34:795–807 Siesjö, BK (1978) Brain energy metabolism. New York, NY: John Wiley & Sons Sonnewald U, Westergaard N, Hassel B, Muller TB, Unsgård G, Fonnum F, Hertz L, Schousboe A, Petersen SB (1993) NMR spectroscopic studies of 13C acetate and 13C glucose metabolism in neocortical astrocytes: Evidence for mitochondrial heterogeneity. Dev Neurosci 15:351–358 Swanson RA (1992) Physiologic coupling of glial glycogen metabolism to neuronal activity in brain. Can J Physiol Pharmacol 70:S138–S144 Swanson RA, Benington JH (1996) Astrocyte glucose metabolism under normal and pathological conditions in vitro. Dev Neurosci 18:515–521 Swanson RA, Yu AC, Chan PH, Sharp FR (1990) Glutamate increases glycogen content and reduces glucose utilization in primary astrocyte culture. J Neurochem 54, 490:496 Swanson RA, Morton MM, Sagar SM, Sharp FR (1992) Sensory stimulation induces local cerebral glycogenolysis: demonstration by autoradiography. Neuroscience 51:451–461 Takahashi S, Driscoll BF, Law MJ, Sokoloff L (1995) Role of sodium and potassium ions in regulation of glucose metabolism in cultured astroglia. Proc Natl Acad Sci U S A 92:4616–4620 Taylor A, McLean M, Morris P, Bachelard H (1996) Approaches to study of neuronal/glial relationships by 13C-MRS analysis. Dev Neurosci 18:434–442 Turecek R, Trussell LO (2000) Control of synaptic depression by glutamate transporters. J Neurosci 20:2054–2063 Tyson RL, Gallagher C, Sutherland GR (2003) 13C-Labeled substrates and the cerebral metabolic compartmentalization of acetate and lactate. Brain Res 992:43–52 Vega C, Martiel JL, Drouhault D, Burckhart MF, Coles JA (2003) Uptake of locally applied deoxyglucose, glucose and lactate by J Cereb Blood Flow Metab, Vol. 24, No. 11, 2004 axons and Schwann cells of rat vagus nerve. J Physiol 546:551– 564 Vicario C, Tabernero A, Medina JM (1993) Regulation of lactate metabolism by albumin in rat neurons and astrocytes from primary culture. Pediatr Res 34:709–715 Voutsinos-Porche B, Bonvento G, Tanaka K, Steiner P, Welker E, Chatton JY, Magistretti PJ, Pellerin L (2003) Glial glutamate transporters mediate a functional metabolic crosstalk between neurons and astrocytes in the mouse developing cortex. Neuron 37:275– 286 Waagepetersen HS, Bakken IJ, Larsson OM, Sonnewald U, Schousboe A (1998) Comparison of lactate and glucose metabolism in cultured neocortical neurons and astrocytes using 13C-NMR spectroscopy. Dev Neurosci 20:310–320 Waagepetersen HS, Sonnewald U, Larsson OM, Schousboe A (2000) A possible role of alanine for ammonia transfer between astrocytes and glutamatergic neurons. J Neurochem 75:471–479 Walz W, Mukerji S (1988) Lactate release from cultured astrocytes and neurons: A comparison. Glia 1:366–370 Wender R, Brown AM, Fern R, Swanson RA, Farrell K, Ransom BR (2000) Astrocytic glycogen influences axon function and survival during glucose deprivation in central white matter. J Neurosci 20:6804–6810 Williams V, Grossman RG, Edmunds SM (1980) Volume and surface area estimates of astrocytes in the sensorimotor cortex of the cat. Neuroscience 5:1151–1159 Wilson JE (1972) The localization of latent brain hexokinase on synaptosomal mitochondria. Arch Biochem Biophys 150:96–104 Wolfe RR (1990) Isotopic measurement of glucose and lactate kinetics. Ann Med 22:163–170 Wolfe RR, Jahoor F, Miyoshi H (1988) Evaluation of the isotopic equilibration between lactate and pyruvate. Am J Physiol 254: E532–E535 Wolff J, Chao TI (2004) Cytoarchitectonics of non-neuronal cells in the central nervous system. In: Non-neuronal cells of the nervous system: Function and dysfunction (Hertz L, ed), Amsterdam: Elsevier, pp 1–52 Wu K, Aoki C, Elste A, Rogalski-Wilk AA, Siekevitz P (1997) The synthesis of ATP by glycolytic enzymes in the postsynaptic density and the effect of endogenously generated nitric oxide. Proc Natl Acad Sci U S A 94:13273–13278 Yager J, Kala G, Hertz L Juurlink BHJ (1994) Correlation between content of high-energy phosphates and hypoxic-ischemic damage in immature and mature astrocytes. Dev Brain Res 82:62–68 Yu ACH, Hertz L (1983) Metabolic sources of energy in astrocytes. In: Glutamine, glutamate and GABA in the central nervous system (Hertz L, Kvamme E, McGeer EG, Schousboe A, eds), New York, NY: Alan R. Liss, pp 327–342 Yu ACH, Lee Y, Eng LF (1992) Glutamate as an energy substrate for neuronal-astrocytic interactions. Prog Brain Res 94:251–259 Yu ACH, Schousboe A, Hertz L (1982) Metabolic fate of 14C-labeled glutamate in astrocytes in primary cultures. J Neurochem 39:954– 960 Zielke HR, Collins RM Jr, Baab PJ, Huang Y, Zielke CL, Tildon JT (1998) Compartmentation of [14C]glutamate and [14C]glutamine oxidative metabolism in the rat hippocampus as determined by microdialysis. J Neurochem 71:1315–1320