Answer Key
advertisement
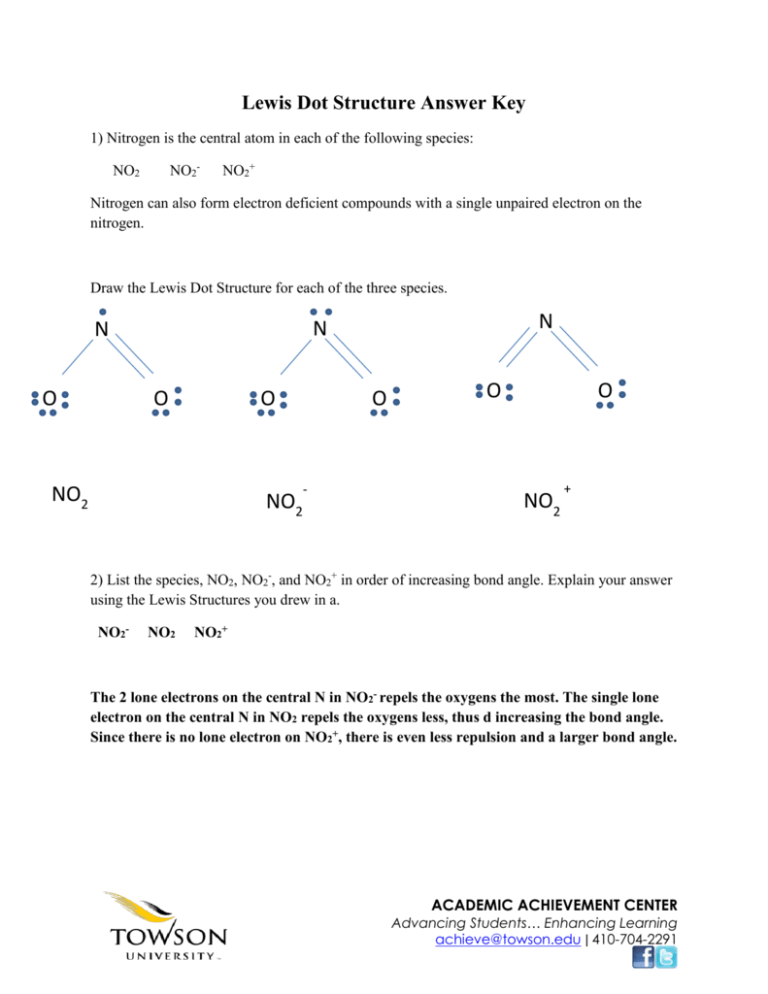
Lewis Dot Structure Answer Key 1) Nitrogen is the central atom in each of the following species: NO2- NO2 NO2+ Nitrogen can also form electron deficient compounds with a single unpaired electron on the nitrogen. Draw the Lewis Dot Structure for each of the three species. O N N N O O NO2 NO2 O - O O NO2 + 2) List the species, NO2, NO2-, and NO2+ in order of increasing bond angle. Explain your answer using the Lewis Structures you drew in a. NO2- NO2 NO2+ The 2 lone electrons on the central N in NO2- repels the oxygens the most. The single lone electron on the central N in NO2 repels the oxygens less, thus d increasing the bond angle. Since there is no lone electron on NO2+, there is even less repulsion and a larger bond angle. ACADEMIC ACHIEVEMENT CENTER Advancing Students… Enhancing Learning achieve@towson.edu ǀ 410-704-2291 3) Give the hybridization and molecular geometry for NO2 NO2 -- --> sp2 hybridized, bent geometry 4) Identify the one species of the three, NO2, NO2-, or NO2+ that dimerizes (reacts with itself) and explain what causes it to do so. NO2 dimerizes to N2O4 because it has a single lone electron and is thus a radical. It has no resonance stability like NO2- or NO2+ 5) Use the principles of bonding to explain the following statement: Phosphorous forms the fluorides PF3 and PF5, whereas nitrogen only forms NF3. Phosphorous can use its d orbitals to bind in addition to its s and p orbitals, thus expanding its shell and allowing it to bond to more fluorines. Since nitrogen can only use its s and p orbitals, it is limited to binding 3 fluorines. 6) Use the principles of bonding to explain the following statement: The SO2 molecule has a dipole moment, whereas the CO2 molecule has no dipole moment The SO2 molecule has a lone pair on its central sulfur, thus making the overall molecule polar and capable of a dipole moment. CO2 has no lone pair on its central carbon, thus making the overall molecule nonpolar and incapable of a dipole moment. 7) Use the principles of bonding to explain the following statement: Molecules of AsF3 are polar, whereas the molecules of AsF5 are nonpolar. AsF3 has a triangular pyramid structure with a lone pair on the central Arsenic.AsF5 has a triangular bipyramidal shape. Arsenic no longer has an lone pair and the overall molecule is thus nonpolar. 8) Use the principles of bonding to explain the following statement: ACADEMIC ACHIEVEMENT CENTER Advancing Students… Enhancing Learning achieve@towson.edu ǀ 410-704-2291 The N--O bonds in NO2- are equal in length, whereas they are unequal in HNO2. The N-O bond in nitrite ion has resonance structure, making the bonds 1.5 instead of a true single and double bond. HNO2 is prevented to have resonance structures by the addition of the hydrogen. 9) Use the principles of bonding to explain the following statement: For sulfur, the fluorides SF2, SF4, and SF6 are known to exist, whereas for oxygen only OF2 is known to exist. Sulfur can use its d orbitals to bind in addition to its s and p orbitals, thus expanding its shell and allowing it to bond to more fluorines. Since oxygen can only use its s and p orbitals, it is limited to binding 2 fluorines. 10) Consider the carbon dioxide molecule, CO2, and the carbonate ion, CO32Draw the Lewis Structure for each. O O C C O CO2 11) The molecular geometry of CO2 is O CO3 O -2 linear ACADEMIC ACHIEVEMENT CENTER Advancing Students… Enhancing Learning achieve@towson.edu ǀ 410-704-2291 12) The molecular geometry of CO32- is trigonal planar 13) Account for the fact that the carbon oxygen bond length in CO3 2- is greater than the carbon oxygen bond length in CO2 The C-O bond in carbonate ion has resonance structure, making the bonds 1.333 instead of a true single and double bonds. CO2 has true double, shorter bond. 14) Consider the molecules CF4 and SF4 Draw the Lewis Structure for each molecule. F F F F C F S F F CF4 SF4 ACADEMIC ACHIEVEMENT CENTER Advancing Students… Enhancing Learning achieve@towson.edu ǀ 410-704-2291 F 15) The molecular geometry of CF4 is tetrahedral 16) The molecular geometry of SF4 is trigonal bipyramidal 17) Account for the fact that CF4 is nonpolar while SF4 is polar SF4 has a lone pair on its central sulfur atom while CF4 does 18) Compare and contrast the three major models (ionic, covalent, and metallic) for bonding. (Hint: What happens to the electrons?) Ionic bonding is between a metal and a nonmetal. Electrons are taken and not shared. Covalent bonding is between a nonmetal and a nonmetal. Electrons are shared between molecules. Metallic bonding is between a metal and a metal. Electrons are delocalized in a sea around the positive nuclei. 19) What are isoelectronic ions? Use electronic configurations to show that S2-, K+, and Ti4+ are isoelectronic. List the three ions in order of INCREASING size. Isoelectric ions are ions with the same electron configuration. S2-, K+, and Ti4+ all have the same electron configuration as Argon: 1s22s22p63s23p6. Increasing size Ti4+, K+, S2- 20) Describe one property of metals. Use the electron sea model to account for this property. ACADEMIC ACHIEVEMENT CENTER Advancing Students… Enhancing Learning achieve@towson.edu ǀ 410-704-2291 Metals have a high melting point. This is because they are held together by metallic bonds. Metallic bonds are a very strong force because the electrons are delocalized in a sea of electrons around the positive nuclei. 21) Compare and contrast the physical properties of ionic and covalent compounds. Covalent compounds have a low polarity while ionic compounds have a high polarity. Covalent compounds share their electrons while ionic compounds take their electrons from other species. Covalent bonds have a low melting/boiling point while ionic compounds have a high melting/boiling point. Covalent compounds are typically liquids or gases at room temperature while ionic compounds are typically solid. ACADEMIC ACHIEVEMENT CENTER Advancing Students… Enhancing Learning achieve@towson.edu ǀ 410-704-2291








