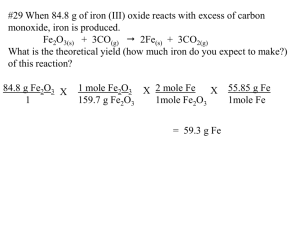
#29 When 84.8 g of iron (III) oxide reacts with excess of carbon
advertisement
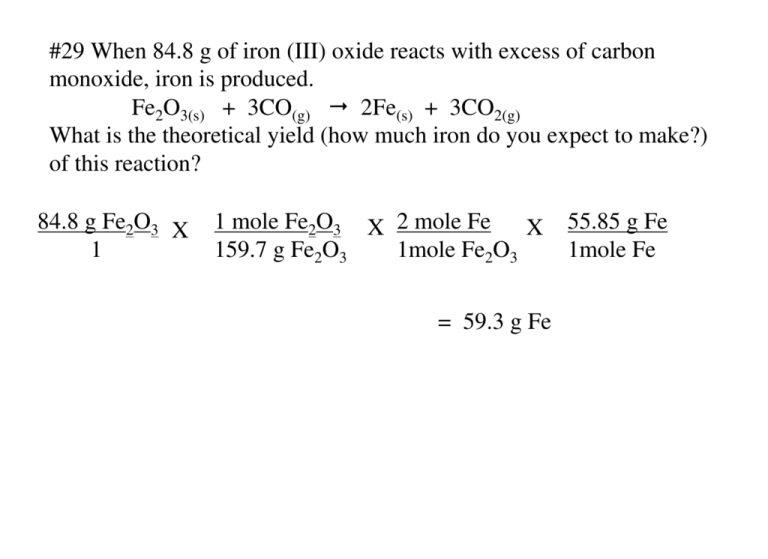
#29 When 84.8 g of iron (III) oxide reacts with excess of carbon monoxide, iron is produced. Fe2O3(s) + 3CO(g) 2Fe(s) + 3CO2(g) What is the theoretical yield (how much iron do you expect to make?) of this reaction? 84.8 g Fe2O3 X 1 1 mole Fe2O3 X 2 mole Fe X 159.7 g Fe2O3 1mole Fe2O3 = 59.3 g Fe 55.85 g Fe 1mole Fe #30 When 5.00 g copper reacts with excess silver nitrate, silver metal and copper (II) nitrate are produced. What is the theoretical yield of silver in this reaction? Cu(s) + 2AgNO3(aq) 2Ag(s) + Cu(NO3)2(aq) 5.00 g Cu 1 X 1 mole Cu 63.55 g Cu X 2 mole Ag 1 mole Cu X = 17.0 g Ag 107.9 g Ag 1 mole Ag #31 If 50.0 g of silicon dioxide is heated with an excess of carbon, 27.9 g of silicon carbide is produced. SiO2(s) + 3C(s) SiC(s) + 2CO(g) What is the % yield in this reaction? First calculate the theoretical yield: 50.0 g SiO2 1 X 1 mole SiO2 60.09 g SiO2 X 1 mole SiC X 1 mole SiO2 40.09 g SiC 1 mole SiC = 33.36 g SiC theoretical 27.9 g SiC X 100 = 83.6 % yield 33.36 g SiC Formula for % yield: actual yield X 100 theoretical yield: #32 If 15.0 g of nitrogen reacts with 15.0 g of hydrogen, 10.5 g of ammonia is produced. What is the percent yield of this reaction? N2 + 3H2 2NH3 calculate the theoretical yield for both nitrogen and hydrogen separately The one that produces the least ammonia determines the theoretical yield, then use that value to determine the % yield: 15.0 g N2 X 1 mole N 17 g NH3 = 2 X 2 mole NH3 X 18.2 g NH3 1 28 g N2 1 mole N2 1 mole NH3 15.0 g H2 X 1 mole H2 X 2 mole NH3 X 17 g NH3 = 85 g NH3 1 2.0 g H2 3 mole H2 1 mole NH3 10.5 g NH3 X 100 = 57.7% 18.2 g NH3 #33 In a chemical reaction, how does an insufficient quantity of a reactant affect the amount of product formed? If a reactant runs out, the reaction must stop. It is like building bicycles, if you run out of one piece you stop building bicycles. #34 How can you gauge the efficiency of a reaction carried out in the laboratory? You can compare your results in the lab with the theoretical results that you can calculate. You can determine the % yield that you got compared with what might be expected with purely calculated values. #35 What is the percent yield if 4.65 g of copper is produced when 1.87 g of aluminum reacts with an excess of copper (II) sulfate? 2 Al(s) + 3 CuSO4(aq) Al2(SO4)3(aq) + 3Cu(s) 1.87 g Al 1 mole Al X X 3 moles Cu X 63.54 g Cu 1 27.0 g Al 2 moles Al 1 mole Cu 4.65 g Cu 6.6 g Cu X 100 = 70.5% Formula for % yield: = 6.6 g Cu theoretical actual yield X 100 theoretical yield: