Balancing Equations Worksheet
advertisement
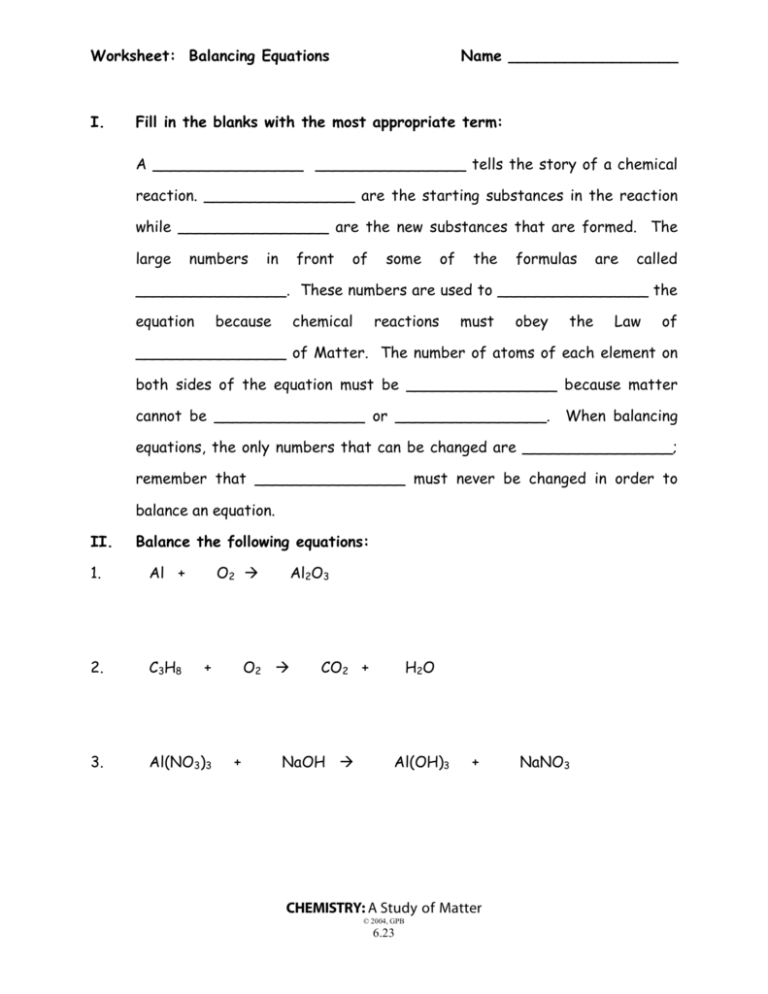
Worksheet: Balancing Equations I. Name __________________ Fill in the blanks with the most appropriate term: A ________________ ________________ tells the story of a chemical reaction. ________________ are the starting substances in the reaction while ________________ are the new substances that are formed. The large numbers in front of some of the formulas are called ________________. These numbers are used to ________________ the equation because chemical reactions must obey the Law of ________________ of Matter. The number of atoms of each element on both sides of the equation must be ________________ because matter cannot be ________________ or ________________. When balancing equations, the only numbers that can be changed are ________________; remember that ________________ must never be changed in order to balance an equation. II. Balance the following equations: 1. Al + O2 2. C3H8 3. Al(NO3)3 + Al2O3 O2 + CO2 + NaOH H2O Al(OH)3 + CHEMISTRY: A Study of Matter © 2004, GPB 6.23 NaNO3 4. KNO3 KNO2 5. O2 6. KClO3 KCl 7. BaF2 K3PO4 8. H2SO4 9. Al + 10. WO3 + + CS2 + + + O2 CO2 + + O2 Ba3(PO4)2 Mg(NO3)2 H2SO4 H2 SO2 KF MgSO4 + Al2(SO4)3 + W + + H2 H2O CHEMISTRY: A Study of Matter © 2004, GPB 6.24 HNO3