51LC EXP #7 SPRING 2012 REACTION: Bromination
advertisement
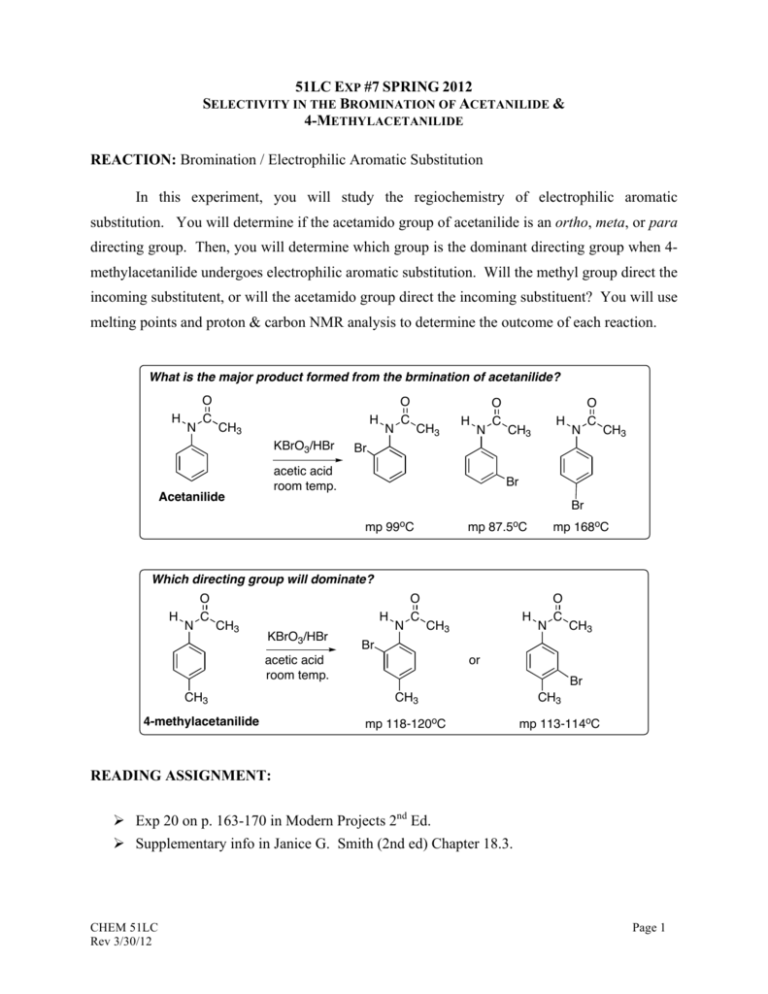
51LC EXP #7 SPRING 2012 SELECTIVITY IN THE BROMINATION OF ACETANILIDE & 4-METHYLACETANILIDE REACTION: Bromination / Electrophilic Aromatic Substitution In this experiment, you will study the regiochemistry of electrophilic aromatic substitution. You will determine if the acetamido group of acetanilide is an ortho, meta, or para directing group. Then, you will determine which group is the dominant directing group when 4methylacetanilide undergoes electrophilic aromatic substitution. Will the methyl group direct the incoming substitutent, or will the acetamido group direct the incoming substituent? You will use melting points and proton & carbon NMR analysis to determine the outcome of each reaction. What is the major product formed from the brmination of acetanilide? H N O C H CH3 KBrO3/HBr N O C CH3 H N H CH3 N O C CH3 Br acetic acid room temp. Acetanilide O C Br Br mp 99oC mp 87.5oC mp 168oC Which directing group will dominate? H N O C CH3 H KBrO3/HBr N O C CH3 4-methylacetanilide N O C CH3 Br acetic acid room temp. CH3 H or Br CH3 mp 118-120oC CH3 mp 113-114oC READING ASSIGNMENT: Ø Exp 20 on p. 163-170 in Modern Projects 2nd Ed. Ø Supplementary info in Janice G. Smith (2nd ed) Chapter 18.3. CHEM 51LC Rev 3/30/12 Page 1 PRE-LAB ASSIGNMENT: Ø How would you expect the IR and NMR spectra to be different between the ortho-, meta, and para-substituted products of acetanilide? Ø Draw all resonance forms that account for the ring substitution of Br group on the acetanilide and 4-methylacetanilide ring system, Based on these resonance structures, and steric factors, explain which product(s) is(are) favored to form. CAUTION Acetanilide and 4-methylacetanilide are toxic and irritants. Avoid all contact with skin, eyes, and clothing, and do all work in an efficient fume hood. Acetic acid is a dehydrating agent and an irritant, and it causes burns. Avoid contact with skin, eyes, and clothing and do all work in an efficient fume hood.. Potassium bromate is a strong oxidant and a cancer suspect agent. No potassium bromate should be discarded in contact with paper because a fire could result. Wet the weighing paper after use and place it in a designated container, not the wastebasket. Hydrogen Bromide solution (48%) and its vapor are corrosive and toxic, and they cause severe burns. Wear gloves and measure the solution in a hood. The reaction mixture contains bromine, the vapors of which are toxic. Be sure that the gas trap is in place during the reaction period. CHEM 51LC Rev 3/30/12 Page 2 EXPERIMENTAL NOTES: Follow the microscale procedure outlined on p. 167 – 169 in Modern Projects 2nd Ed. As part of you in-lab work, analyze both the IR and NMR spectra for the bromination products of acetanilide and 4-methylacetanilide. These are available on the course website. The most important one is the 1H NMR spectra. Use Table 21.4 in Techniques 3rd Ed. to calculate the chemical shifts for the two possible regioisomers of the product (read the Note below for helpful information). You should include these in your in-lab observation with brief analysis. They will help you determine which bromination product was obtained. You should discuss any changes in the spectra for the starting material vs. the product (appearance/disappearance of any peaks, changes in the number of nonequivalent H's, changes in the aromatic region, etc.) NOTE: Predicting the NMR shifts of protons is a very useful technique, but the predicted values don’t always match the experimental ones. Sometimes, in fact, they are quite off. The relative values, however are usually fairly reliable. In other words, even though the actual ppm values are wrong the order of the peaks will be correct. When you calculate the predicted values, draw a picture of what the aromatic region of the spectra for each regioisomers will look like – include the splitting (doublets or singlets). Now compare that picture to the spectrum of the actual product. The one that looks more similar (the peaks are in the correct order) is the correct product. CHEM 51LC Rev 3/30/12 Page 3