Flammability issues of mixtures: If you hold a match over pure MeOH
advertisement
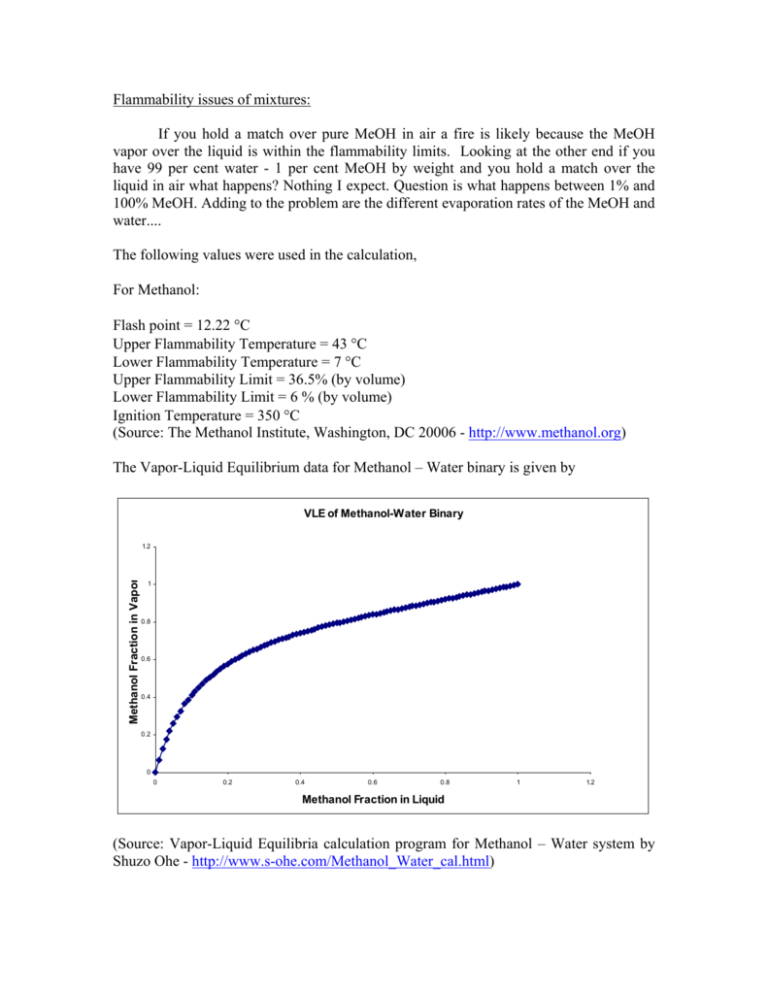
Flammability issues of mixtures: If you hold a match over pure MeOH in air a fire is likely because the MeOH vapor over the liquid is within the flammability limits. Looking at the other end if you have 99 per cent water - 1 per cent MeOH by weight and you hold a match over the liquid in air what happens? Nothing I expect. Question is what happens between 1% and 100% MeOH. Adding to the problem are the different evaporation rates of the MeOH and water.... The following values were used in the calculation, For Methanol: Flash point = 12.22 °C Upper Flammability Temperature = 43 °C Lower Flammability Temperature = 7 °C Upper Flammability Limit = 36.5% (by volume) Lower Flammability Limit = 6 % (by volume) Ignition Temperature = 350 °C (Source: The Methanol Institute, Washington, DC 20006 - http://www.methanol.org) The Vapor-Liquid Equilibrium data for Methanol – Water binary is given by VLE of Methanol-Water Binary Methanol Fraction in Vapor 1.2 1 0.8 0.6 0.4 0.2 0 0 0.2 0.4 0.6 0.8 1 1.2 Methanol Fraction in Liquid (Source: Vapor-Liquid Equilibria calculation program for Methanol – Water system by Shuzo Ohe - http://www.s-ohe.com/Methanol_Water_cal.html) The Vapor Pressure vs. Temperature plot for methanol is (Source: Vapor Pressure ohe.com/methanol.html) Data for Methanol, Shuzo Ohe – http://www.s- The VLE data of Methanol – Water Binary was fitted to the following equation, y = −16.695 x 6 + 58.50 x 5 − 82.079 x 4 + 59.404 x 3 − 23.978 x 2 + 5.824 x + 0.133 where ‘y’ is the mole fraction of methanol in the vapor phase and ‘x’ is the mole fraction of methanol in the liquid phase. Similarly, the Vapor Pressure vs. Temperature data of methanol was also fitted to the following equation, V .P = 41.52e 0.0427T where ‘V.P’ is the vapor pressure of methanol at temperature ‘T’. Using the VLE data and the Vapor Pressure Data, composition of methanol vapor over the methanol – water binary was calculated for various temperatures and the plot is given below, For example, at 30 °C, a composition of 6% Methanol and up would produce a combustible vapor above its surface and if there is a source near the surface whose temperature is more than the ignition temperature (350 °C) of methanol, fire is likely. 2. Given a round gallon can of liquid open to air. Start with a 50/50 mix and show what happens to concentration and volume with time. Also, seal a half full can. What is the pressure and composition of the vapor at say 150 F. Let us take a mixture of 2 moles of methanol (64g) and 2 moles of water (36g) in a round gallon can whose radius is 5 cms. Let us assume that the temperature and pressure are 65.5 °C (150 F) and 1 atm respectively. The plot mole fraction of methanol vs. time is given below followed by the total volume of the mixture vs. time, If the can were sealed, the pressure of the methanol vapor would be the equal to the vapor pressure of methanol at 150 F (65.5 °C) times the mole fraction of methanol in vapor as given by the VLE data. The pressure in this case would be 0.8343 atm and composition of the vapor would be 79.26% methanol and the rest water vapor.







