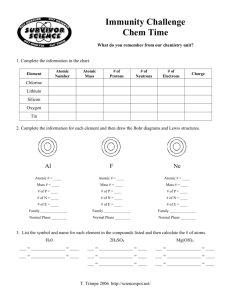
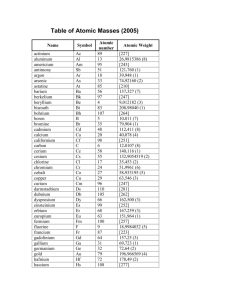
p.278 - Bryn Mawr School Faculty Web Pages
advertisement

", J , '/ ,' / Cumulative Standardized Test Practice or SiI ~ is tetraiodosilane, What is its molecular compound name? A . silane tetraiodide B. silane tetraiodine C. silicon iodide D. silicon tetraiodide 1. The common name 6. Th e centra l selenium atom in selenium hexafluo­ ride (arms an expanded octet. How man y electron pairs surround the central Se atom? A . 4 C. 6 B. 5 D. 7 Use the table below 10 answer Questions 7 and 8. I 2. Which compound contains at least one pi bond? ( CO~ A. B. CHCl 3 C. As!} D. Bef 2 Use th e graph belo w to answer Q uestio ns 3 and 4. 345 N""N O-H 416 (-0 358 (=0 745 0=0 498 3Q5 5 >- 4 ... :~ H- I 299 H-N 391 -- 13 «i 3 ~ a.o c: e t 7. Which diatomic gas has the shortest bond between its two atoms? A. HI B. O 2 2 a.o WI o I - - - II II 1 2 3 4 5 6 7 8 9 10 111213 14151617 181920 8 . Approximately how much energy will it take to break all the bonds present in the molecule below? Atomic number 3. What is the electronegat ivity of the element with atomic numb er 14? A. I.S B. 1.9 C. 2.0 D.2 .2 H number number number number 3 and 7 and 4 and 8 and atomic atomic atomic atomic number number number number · . .. ·. c. ·S : 5i : 5. D. : S : Si:S : 278 Chapter 8 • Assessment "/ H-( HI A. B. C. D. 4 8 J8 12 3024 4318 4621 5011 c 1" r H ( 0 >. H 0" klimat kl/rnol kl/rnol kl/rnol 9. Which compound does NOT have a bent molecular shape? A. BeH z B. HzS 5. Wh ich is the Lewis structure for silicon disulfide? A. : 5:: Si :: 5: B. 5 : :5 i:: 5 H 1 H 4. An ionic bond would form between which pairs of elem ents? A. atomic B. atomic C. atomic D . atomic "N/ 10. Which compound is nonpola r? A. HzS C. SiH 3CI B. CCl 1 D . AsH 3 ~ Chemi~'1 l i "lS _L _ _ -­ Standardized Test Practice p~~ 14.