Chapter 1
advertisement
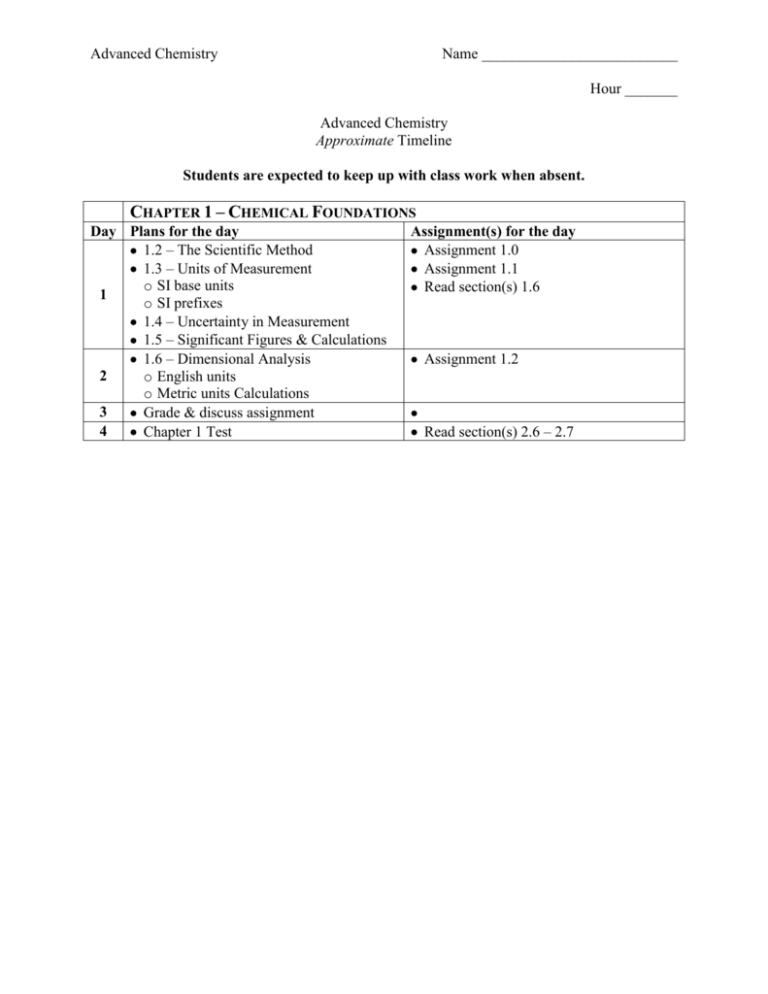
Advanced Chemistry Name __________________________ Hour _______ Advanced Chemistry Approximate Timeline Students are expected to keep up with class work when absent. CHAPTER 1 – CHEMICAL FOUNDATIONS Day Plans for the day 1.2 – The Scientific Method 1.3 – Units of Measurement o SI base units 1 o SI prefixes 1.4 – Uncertainty in Measurement 1.5 – Significant Figures & Calculations 1.6 – Dimensional Analysis 2 o English units o Metric units Calculations 3 Grade & discuss assignment 4 Chapter 1 Test Assignment(s) for the day Assignment 1.0 Assignment 1.1 Read section(s) 1.6 Assignment 1.2 Read section(s) 2.6 – 2.7 Advanced Chemistry Name __________________________ Hour _______ Study Guides Chapter 1 Quizzes Advanced Chemistry Quiz 1.1 – 1.2 1.2 1. 2. 1.3 The Scientific Method Know the general frame work of the scientific method (three steps). Know the difference between a theory and a law. Units of Measurement Know the SI base units of: a. mass b. length c. time d. temperature e. amount of substance 4. Know the prefixes used in the SI/metric system. (symbol, meaning, exponential notation) a. mega b. kilo c. deci d. centi e. milli f. micro g. nano 3. Quiz 1.4 –1.5 1.4 Uncertainty in Measurement 5. The uncertainty in a measurement depends upon ___. 6. Know and understand the definitions of the terms accuracy and precision 1.5 Significant Figures and Calculations 7. Know the rules for counting significant figures. 8. Be able to indicate the number of significant figures in a measurement. 9. Perform a simple calculation and round the answer to the correct number of significant figures. Quiz 1.6 1.6 10. 11. Dimensional Analysis Know the general process for converting from one unit to another. Use dimensional analysis to perform calculations Study Guide Chapter 1 Test Advanced Chemistry At the completion of chapter 1 you should… 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. Know the definitions of the following terms. a. Hypothesis b. Mass c. Weight d. Accuracy e. Precision f. Density g. Homogeneous mixture h. Heterogeneous mixture i. Physical change j. Chemical change k. Element l. Compound Know the SI base units. Know the prefixes used in the SI system of measurement. Be able to indicate the number of significant figures in a measurement. Be able to handle calculations involving significant figures. Use dimensional analysis to perform unit conversions. Be able to perform temperature conversions. Be able to solve density problems. Be able to classify matter as… a. an element b. a compound c. a homogeneous mixture (solution) d. a heterogeneous mixture Be able to classify changes as… a. a chemical change b. a physical change Advanced Chemistry Name __________________________ Hour _______ Assignment 1.0 – Vocabulary Define each of the following terms. 1. Hypothesis 2. Mass 3. Weight 4. Density 5. Homogeneous mixture 6. Heterogeneous mixture 7. Physical change 8. Chemical change 9. Element 10. Compound 11. Accuracy 12. Precision Advanced Chemistry Name __________________________ Hour _______ Assignment 1.1 – Significant Figures 1) 2) 3) Indicate the number of significant figures in each of the following: A) This book contains more than 1000 pages. ____________ B) A mile is about 5300 ft. ____________ C) A liter is equivalent to 1.059 qt. ____________ D) The population of the United States is more than 3.0 x 102 million. ____________ E) A kilogram is 1000 g. ____________ F) A jet airplane cruises at around 600 mi/h. ____________ How many significant figures are in each of the following? A) 100 ____________ B) 1.0 x 102 ____________ C) 1.00 x 103 ____________ D) 100. ____________ E) 0.0048 ____________ F) 0.00480 ____________ G) 4.80 x 10-3 ____________ H) 4.800 x 10-3 ____________ Perform the following mathematical operations and express the result to the correct number of significant figures. _____________ A) B) 0.14 x 6.02 x 1023 __________________ C) 4.0 x 104 x 5.021 x 10-3 x 7.34993 x 102 __________________ _____________ D) E) 6.022 x 1023 x 1.05 x 102 __________________ _____________ F) G) 1.285 x 10-2 + 1.24 x 10-3 + 1.879 x 10-1 __________________ H) 1.285 x 10-2 - 1.24 x 10-3 __________________ I) _____________ J) (100 is exact) __________________ K) (3 is exact) __________________ Advanced Chemistry Name __________________________ Hour _______ Assignment 1.2 – Unit Conversions Show all work. No work = no credit. You will need your book to look up equivalent measures. (page A26) 1) Perform the following unit conversions. A) 908 oz to kilograms. B) 12.8 L to gallons C) 125 mL to quarts D) 2.89 gallons to milliliters E) 4.48 lb to grams F) 550 mL to quarts 2) Using the following exact equivalence statements to perform the stated calculations: 5 ½ yards = 1 rod 40 rods = 1 furlong 8 furlongs = 1 mile The Kentucky Derby race is 1.25 miles. How long is the race in: A) rods? B) furlongs? C) meters? D) kilometers?






