Chemical Reactions
advertisement

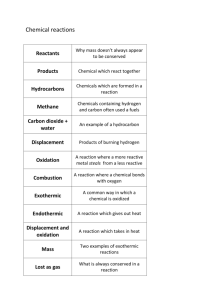
Unit 2: Chemical Reactions Chapter 4: The Effects of Chemical Reactions Section 4.1: Introduction to Chemical Reactions, pages 53–54 1. A physical change in a substance occurs when a substance changes its form or state. Physical change does not require a change in the chemical identity of the substance. 2. A chemical change in a substance occurs when the substance is changed into a different substance. 3. Table 1 Chemical and Physical Changes The reaction Type of change that occurred Water is heated in a pot on the stove and becomes vaporized. physical change Water and metal react to form rust. chemical change A snowball melts in your hand. physical change Hydrogen peroxide is mixed with water to form hydrogen and chemical change oxygen gas. 4. A change in colour, the release of a gas, the release of thermal energy, and the formation of a solid residue are all clues that a chemical reaction has taken place. 5. (a) No. A chemical change does not occur when water is boiled on a stove, producing a gas. (b) To prove this, the product produced (the gas) must be tested to see if it is chemically different from the starting material (the water). 6. The solid produced in the reaction liquid + liquid → solid is called a precipitate. 7. Table 2 Identification of Materials Chemical Description CH2CH2OH reactant HCl reactant H2SO4 catalyst CH3CH2Cl product H2O product 8. The concept that in a chemical reaction the mass of the reactants equals the mass of the products is known as the law of conservation of mass. 9. Table 3 Identification of State Symbol State symbol Meaning (s) solid (l) liquid (g) gas (aq) aqueous 10. Table 4 Balancing Equations word equation water → hydrogen + oxygen skeleton equation H2 O(l) → H2(g) + O2(g) balanced chemical equation 2 H2O(l) → 2 H2(g) + O2(l) 11. (a) CH4(g) + 2 O2(g) → CO2(g) + 2 H2O(l) (b) 2 Mg(s) + O2(g) → 2 MgO(s) (c) 2 Fe(s) + 3 Cl2(g) → 2 FeCl3 (aq) (d) 2 C2 H6(g) + 7 O2(g) → 4 CO2(g) + 6 H2O(l) Copyright © 2011 Nelson Education Ltd. Chapter 4: The Effects of Chemical Reactions 4-1 Section 4.2: Synthesis and Decomposition Reactions, pages 55–57 1. b 2. Answers may vary but should include two of the following: lithium, sodium, rubidium, or cesium. Sample answer: Based on potassium’s reactivity with chlorine, two other elements that will react with chlorine are lithium and sodium. 3. In a synthesis reaction, two reactants collide, breaking the existing bonds between their atoms, and form new bonds. In this process, a new compound is formed. The product is typically larger and more complex. 4. Table 1 Synthesis Reaction for Sodium and Oxygen word equation sodium + oxygen → sodium oxide Na(s) + O2 (g) → Na2 O(s) skeleton equation 4 Na(s) + O2(g) → 2 Na2O(s) 5. False. Group 1 elements, which include alkali metals, typically form ionic compounds. (Hydrogen forms molecular compounds.) 6. Molecular compounds are made up of atoms. 7. Table 2 Completing and Balancing Equations for Synthesis Reactions Reactants Product Balanced equation N2(g) + H2(g) NH3(g) N2(g) + 3 H2(g) → 2 NH3 (g) balanced chemical equation Li(s) + Cl2(g) LiCl(s) 2 Li(s) + Cl2(g) → 2 LiCl(s) Al(s) + Br2(g) AlBr3(s) 2 Al(s) + 3 Br2(g) → 2 AlBr3(s) 2 K(s) + S(s) → K2S(s) 8. Synthesis reactions with can be difficult to predict when they are a reaction between non-metals other than hydrogen because the products of these reactions often depend on the reaction conditions. 9. d 10. True 11. In a decomposition reaction, a larger or more complex compound breaks down to form two (or more) simpler products. 12. In a synthesis reaction, two reactants combine to make a larger or more complex product. Decomposition reactions occur in the opposite manner. One larger or more complex reactant breaks down to form two (or more) simpler products. 13. Table 3 A Decomposition Reaction word equation hydrogen chloride→ hydrogen + chloride K(s) + S(s) K2S(s) skeleton equation HCl(g) → H2 (g) + Cl2(g) 2 HCl(g) → H2(g) + Cl2 (g) 14. Table 4 Completing and Balancing Equations for Decomposition Reactions Reactant Products Balanced equation Ni2O3(s) Ni(s) + O2(g) 2 Ni2O3(s) → 4 Ni(s) + 3 O2(g) balanced chemical equation Al2O3(s) Al(s) + O2(g) 2 Al2O3(s) → 4 Al(s) + 3 O2(g) Ca3P2(s) Ca(s) + P(s) Ca3P2(s) → 3 Ca(s) + 2 P(s) Na2CO3 (s) Na2O(s) + CO2(g) Na2CO3(s) → Na2O(s) + CO2(g) 15. Table 5 Types of Reactions Equation H2CO3(aq) → H2O(l) + CO2(g) Type of Reaction decomposition 2 Mg(s) + 2 O2(g) → 2 MgO(s) synthesis H2SO3(aq) → H2O(l) + SO2(g) decomposition 2 P(s) + 3 Cl2(g) → 2 PCl3(g) synthesis Copyright © 2011 Nelson Education Ltd. Chapter 4: The Effects of Chemical Reactions 4-2 Section 4.3: Explore an Issue in Chemical Reactions: Garbage Gasification —A Heated Debate, page 58 1. Gasification is heating waste to temperatures high enough to cause the molecules of the waste substances to decompose into simpler substances. 2. The transformation of large compounds into the component parts of syngas represents decomposition. 3. (a) Using syngas to create methanol, CH3OH(g), is a synthesis reaction. (b) Skeleton equation for this reaction: H2(g) + CO(g) → CH3OH(g) (c) Balanced equation for this reaction: 2 H2(g) + CO(g) → CH3OH(g) 4. Steps involved in the critical thinking process for determining the solution for an environmental problem include research, determination of benefits and drawbacks of possible solutions, identification of alternatives, comparison of solutions, decision making, and communication. 5. (a).Table 1 Benefits and Drawbacks of Gasification Benefits of gasification Drawbacks of gasification • safe • produces harmful emissions that may damage human • produces energy that can be sold health • cost-effective • may undermine recycling progress already made • addresses the problem of increasing quantities • unknown environmental risks of waste (b) The hazardous materials created by the process of gasification include acids, heavy metals, and ash. Section 4.4: Single Displacement Reactions, pages 59–61 1. Single displacement reactions often are used when metals need to be recovered from a solution. 2. (a) Table 1 A Single Displacement Reaction word equation zinc + copper chloride → zinc chloride + copper skeleton equation Zn(s) + CuCl2(l) → ZnCl2(l) + Cu(s) Zn(s) + CuCl2(l) → ZnCl2(l) + Cu(s) (b) The solid copper formed in this reaction is known as a precipitate. 3. (a) Reactants (given): Mg(s) + HCl(l) Products: MgCl(s) + H2(g) Balanced equation: 2 Mg(s) + 2 HCl(l) → 2 MgCl(s) + H2(g) (b) Reactants (given): Zn(s) + H2SO4(l) Products: ZnSO4(l) + H2(g) Balanced Equation: Zn(s) + H2SO4(l) → ZnSO4 (l) + H2 (g) (c) Reactants (given): Cu(s) + AgNO3(aq) Products: Ag(s) + Cu(NO3)2(aq) Balanced Equation: Cu(s) + 2 AgNO3(aq) → 2 Ag(s) + Cu(NO3)2(aq) 4. An activity series is a ranking of the relative reactivity of metals or halogens in single displacement reactions. The more reactive the element, the higher it is listed in the activity series. An elements can displace any elements below it in the activity series. 5. False. The most reactive metals are at the top of the activity series. 6. Two important generalizations about activity series are: 1) An element higher on the list is able to replace any element below it on the list. 2) The farther apart two elements are in an activity series, the more likely it is that the displacement reaction will occur quickly. balanced chemical equation Copyright © 2011 Nelson Education Ltd. Chapter 4: The Effects of Chemical Reactions 4-3 7. (a) Potassium will replace sodium in a single displacement reaction because it is more reactive than sodium. (b) Platinum will not replace chromium in a single displacement reaction because platinum is less reactive than chromium. (c) Table 2 Single Displacement Reactions Equation Products of Reaction no reaction Al2O3(aq) + 2 Fe(s) → 2 Al(s) + Fe2O3(aq) → Al2O3(aq) + 2 Fe(s) Cl2(g) + 2 KBr(aq) → 2 KCl(aq) + Br2(l) no reaction Al(s) + MgCl2(aq) → 8. a 9. A different pattern is used to predict the reactivity of halogens because displacement of a halogen involves the displacement of a negative ion (anion) rather than a positive ion (cation). 10. (a) Cl2(aq) + 2 NaBr(aq) → Br2(aq) + 2 NaCl(aq) (b) Br2(g) + 2KI(s) → 2 KBr(s) + I2(g) (c) Br2(g) + 2 NaCl(aq): No reaction occurs between these reactants because bromine is lower on the activity series for the halogens than chlorine. 11. Sodium hydrogen carbonate, NaHCO3, is effective for neutralizing stomach acid, HCl, because hydrochloric acid reacts in a displacement reaction with sodium hydrogen carbonate to form sodium chloride, water, and carbon dioxide. The sodium replaces the hydrogen in the hydrochloric acid, forming sodium chloride, or table salt. This reduces the quantity of stomach acid present and the painful burning sensation caused during indigestion. Section 4.5: Chemistry Journal: The Mystery of the Missing Mercury, page 62 1. Mercury disappears from the Arctic air during the spring when highly reactive bromine atoms, Br0, in seawater react with oxygen in the air to produce bromine monoxide, BrO. Bromine monoxide is also highly reactive. Scientists think both bromine and bromine monoxide react with mercury in the air, pulling away two of its electrons to leave a mercury(II) ion, Hg2+, that can attaches itself to snowflakes and fall to the ice surface. 2. Mercury remains in the environment for a long time because it has a very low level of reactivity. Looking at the reactivity series, it is evident that there are few elements that would be capable of replacing the element in a displacement reaction. In most instances, mercury will come into contact with compounds that contain elements that are more reactive. 3. Large amounts of mercury can be found in our environment because the combustion of coal releases mercury into the atmosphere. Small quantities of mercury also enter the atmosphere from volcanic activity. Wind can transport mercury in the atmosphere all over the globe. 4. Some of the challenges that exist for furthering research regarding the missing mercury in the Arctic are the huge size of the Arctic, a landscape that is difficult to navigate, the extreme climate, plus the costs involved in overcoming these challenges. 5. Mercury attached to carbon atoms poses a unique threat to human and animal health because it is more likely to enter the bodies of organisms and to bioaccumulate. Once in the body, it can harm the nervous system, because it is a potent neurotoxin. Copyright © 2011 Nelson Education Ltd. Chapter 4: The Effects of Chemical Reactions 4-4 Section 4.6: Double Displacement Reactions, pages 63–64 1. c 2. False. A substance is described as very soluble if a significant quantity of the substance dissolves. 3. Table 1 A Double Displacement Reaction for Calcium Carbonate and Hydrochloric Acid word calcium carbonate + hydrochloric acid → calcium chloride + water + carbon dioxide equation skeleton CaCO3(s) + HCl(l) → CaCl2(s) + H2 O(l) + CO2 (g) equation balanced CaCO3(s) + 2 HCl(l) → CaCl2 (s) + H2 O(l) + CO2(g) chemical equation 4. (a) Reactants (given): KOH(l) + H2SO4(l) Products:K2SO4(s)+ H2O(l) Balanced equation: 2 KOH(l) + H2SO4(l) → K2SO4(s)+ 2 H2O(l) (b) Reactants (given): FeS(s) + HCl(l) Products: FeCl2(s)+ H2S(g) Balanced Equation: FeS(s) + 2 HCl(l) → FeCl2(s)+ H2S(g) (c) Reactants (given): NaCl(s) + H2SO4(l) Products: Na2SO4(s)+ HCl(l) Balanced Equation: 2 NaCl(s) + H2SO4(l) → Na2SO4(s)+ 2 HCl(l) 5. In the equation NaCl(s) + H2O(l) → Na–(aq) + Cl+(aq), NaCl is the solute and H2O is the solvent. 6. Table 2 Double Displacement Reactions Equation Products of Reaction no reaction CaBr2 + 2 KOH → Cu(OH)2 + 2 HC2H3O2 → Cu(C2 H3O2) 2 + 2 H2 O FeS + 2 HCl → FeCl2 + H2S no reaction Ca(NO3)2 + 2 HCl → 7. Cl and NO3 are both highly soluble. NO3 will have no exceptions. However, Cl is not highly soluble when it is attached to Ag. Thus, while it may be possible for the KNO3 to dissolve into its anion and cation parts, AgCl will not be soluble, preventing a reaction from occurring. 8. H2CO3(aq) → H2O(l) + CO2(g) 9. A neutralization reaction creates a solution in which the pH of the final product is closer to 7 (neutral) than the pH of either of the reactants. A reaction can be confirmed by measuring the pH of the final solution and comparing it with the pH of the reactant solutions. 10. (a) HCl(l) + NaOH(aq) → NaCl(s)+ H2O(l) (b) H2SO4 (l)+ 2 NH4OH(l) → (NH4)2SO4 (l)+ 2 H2O(l) (c) 2 NaOH(aq) + H2CO3 (l) → Na2CO3(aq) + 2 H2O(l) Copyright © 2011 Nelson Education Ltd. Chapter 4: The Effects of Chemical Reactions 4-5 Chapter 4 Summary, page 65 Copyright © 2011 Nelson Education Ltd. Chapter 4: The Effects of Chemical Reactions 4-6 Chapter 4 Questions, pages 66–67 1. d 2. a 3. (a) True (b) False. In a neutralization reaction, no precipitate is formed. 4. Single displacement reactions involve the displacement of one element in a compound to produce one new element and one new compound. Double displacement reactions involve the displacement of two elements in two different compounds, producing two new compounds. 5. A single displacement reaction will not occur between Al(s) and MgCl2(aq) because magnesium is more reactive than aluminum. If the more reactive element is attached in the compound, the less reactive element cannot react. 6. A double displacement reaction will not occur between calcium bromide, CaBr2, and potassium hydroxide, KOH, because the products of the reaction do not include water, a gas, or a precipitate. Both of the products formed in the reaction, calcium hydroxide, Ca(OH)2, and potassium bromide, KBr, are highly soluble and will remain in the aqueous form. 7. (a) A chemical reaction has occurred if energy is released, the solution changes colour, or a gas is released. (b) Another observable change indicating that a chemical reaction has occurred is that a precipitate has formed. (c) If no observable change occurred, the pH of the solution could be measured and compared to the pH of each reactant to determine the presence of a chemical reaction. 8. Table 1 Identification of Materials Chemical Description 2 SO3 product 2 SO2 reactant V2O5 catalyst 9. Table 2 Classification of Reactions Equation NaCO3(s) → NaO(s) + CO2(g) Type of Reaction decomposition Zn(s) + CuCl2(l) → ZnCl2 (l) + Cu(s) single displacement 4 Na(s) + O2(g) → 2 Na2O(s) synthesis CaCO3 (s) + 2 HCl(l) → CaCl2(s) + H2 O(l) + CO2(g) double displacement 2 KOH(l) + H2SO4 (l) → K2SO4 (s)+ 2 H2O(l) double displacement Copyright © 2011 Nelson Education Ltd. Chapter 4: The Effects of Chemical Reactions 4-7