Journal of Chemical and Pharmaceutical Research, 2012, 4(6):3171
advertisement

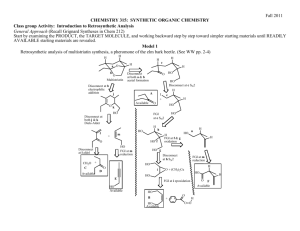
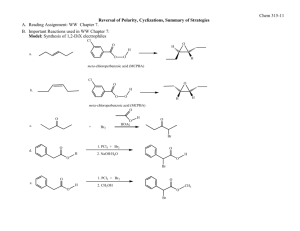
Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2012, 4(6):3171-3183 Research Article ISSN : 0975-7384 CODEN(USA) : JCPRC5 Synthesis Design of ‘Rivastigmine’-A Potent Therapeutic Agent for Alzheimer’s disease using Retrosynthetic Analysis Chittaranjan Bhanja* and Satyaban Jena Department of Chemistry, Utkal University, Bhubaneswar-751004, Odisha, India _____________________________________________________________________________________________ ABSTRACT Synthesis is an indispensable component in the multidisciplinary development process of small molecule pharmaceuticals. Designing synthetic routes to the pharmaceuticals by retrosynthetic analysis /synthon disconnection approach, developed by Prof.E.J.Corey of Harvard University has emerged as powerful means in synthetic organic/medicinal chemistry. Keeping a bird’s eye view on the works published in journals and patent literatures, some synthesis schemes have been proposed for a potent Alzheimer’s disease drug ‘Rivastigmine’ in a novel way basing on the retrosynthetic analysis. The proposed synthesis planning being a theoretical exploration, the actual laboratory execution requires the cross examination of a considerable number of factors such as reactions, reagents and order of events. In actual practice, generally that route is most feasible which satisfies the specific criterion for an ideal synthesis. Key words: Acetyl cholinesterase inhibitor, Alzheimer’s disease, Retrosynthetic analysis, Rivastigmine, Synthesis, Synthon disconnection approach _____________________________________________________________________________________________ INTRODUCTION Organic synthesis is not only the fundamental tool to find essential drug candidate molecules but also in charge of the subsequent creation, exploration and evaluation of short, efficient, safe and economically viable synthetic routes for the selected clinical candidates. The heart of any synthesis is the planning of synthetic routes to a molecule of any interest. If there is any key to success in planning a synthesis, it is to work the problem in the backward/ reverse direction of synthesis, called retrosynthetic analysis/synthon disconnection approach[1,2], a strategy developed systematically by Noble Laureate Prof. E.J.Corey of Harvard University. By working backwards the aim of the retrosynthetic analysis is to transform a synthesis target in to progressively simpler structures by making strategic bond cleavages and functional group interconversions, following a pathway to commercially available starting materials. Analysis of a target molecule in retrosynthetic direction usually results a large number of possible synthetic routes. It is therefore necessary to critically assess each derived route in order to choose the single route which meets the specific criterion for an ideal synthesis. Alzheimer’s disease (AD) is an irreversible, complex, neurodegenerative disorder characterized by progressive cognitive impairment, a variety of neuropsychiatric and behavioral disturbances and restrictions in activities of daily life [3]. It is an age related disease and is the most common cause of dementia in old people, being diagnosed after the age of 56, and affecting up to 10 % of the population over the age of 65. The disease affects 30% or more of the population over the age of 80. In the developed world, AD is the fourth major cause of death after cardiovascular disease, cancer, and cerebral accidents. With an increase in life expectancy due to medical advances in the treatment 3171 Chittaranjan Bhanja et al J. Chem. Pharm. Res., 2012, 4(6):3171-3183 ______________________________________________________________________________ of the above diseases, the number of AD patients is anticipated to increase dramatically. Worldwide, there are approximately 35 million people with AD, and that number is expected to grow to 107 million by 2050 [4]. The etiology of AD is not known, however, the biochemical and pathophysiological findings on postmortem brain examination of AD patients have shown that the loss of the basal forebrain cholinergic system is one of the most significant aspects of neurodegeneration in the brains of AD patients, and it is thought to play a central role in producing cognitive impairments [5–7]. One of the therapeutic strategies aimed at ameliorating the clinical manifestations of Alzheimer’s disease is to enhance cholinergic neurotransmission in relevant parts of the brain by the use of acetyl cholinesterase inhibitors to delay the breakdown of acetylcholine released into synaptic clefts. Therefore, enhancement of cholinergic transmission has been regarded as one of the most promising methods for treating AD patients. A number of drugs are presently in clinical trials for the treatment of AD and among the best known of these are the acetyl cholinesterase inhibitors .Rivastigmine,(S)-3-[1-(dimethylamino) ethyl] phenyl ethyl (methyl) carbamate I, with a phenylcarbamate structure, is the first U.S.FDA approved drug for the treatment of mild to moderate dementia of the Alzheimer’s type[8-11]and for mild to moderate dementia related to Parkinson’s disease[12].It works by blocking the acetylcholine esterase (AChE), the enzyme responsible for its degradation and butyrylcholine esterase (BuChE), the enzyme responsible for hydrolysis of ACh, thereby increasing both the level and duration of action of the neurotransmitter acetylcholine[13-14]. In particular, Rivastigmine appears to have marked effects in patients showing a more aggressive course of disease, such as those with a younger age of onset or a poor nutritional status, or those experiencing symptoms such as nausea and vomiting. The high potentiality of Rivastigmine against Alzheimer’s disease deserves an appropriate position in the “Blockbuster Drug List”[15]. N N O O I: Rivastigmine Few synthetic methodologies for Rivastigmine although well cited in the literature[16-34 ],some alternative synthetic routs and improvement in its existing processes for manufacture are constantly required in pharmaceutical industries for market development. Keeping a bird’s eye view on the published works both in journals and patent literatures, we have focused our research attention to propose a good number of synthesis schemes for ‘Revastigmine’ based on retrosynthetic analysis / synthon disconnection approach . It is an innovative work that has not been reported elsewhere. The choice of this molecule for synthesis planning is obvious as Alzheimer’s disease is worlds’ 4th most prevalent, debilitating and progressive disease that covers more than 35 millions people world wide and Rivastigmine is one of the potent acetyl cholinesterase inhibitor widely used for the treatment of mild to moderate dementia of the Alzheimer’s type as well as Parkinson’s disease. EXPERIMENTAL SECTION The structure and information regarding Rivastigmine as drug candid has been collected from different books.[1-4 ]. The proposed synthesis planning are then exploited in a novel way from the result of retrosynthetic analysis of dug structure using the basic principle outlined in the pioneering works of Prof. E.J. Corey. The terms, abbreviations and symbols used during synthesis planning are synonymous to that represented in book. [5].The analysis–synthesis schemes being theoretical propositions, obviously the synthesis have not been executed in the laboratory. Most of the retrosynthesis schemes have been derived taking in to account the synthesis earlier done for its preparation as found from different literatures. The actual laboratory execution requires the cross examination of a considerable number of factors such as reagents, reactions, order of events, economical viability, environmental benign, saftyness, short time and scalable synthesis. 3172 Chittaranjan Bhanja et al J. Chem. Pharm. Res., 2012, 4(6):3171-3183 ______________________________________________________________________________ RESULTS AND DISCUSSION Retrosynthetic Analysis-1 N N O N C-O O TM N N Cl HO O 1 O-carbamoylation MeO FGI + de-methylation 2 5 C-N C-N NH + Cl3O 3 O OH O FGI MeO MeO NH OCl3 4 + 6 H 8 7 Synthesis-1 O MeO NH,benzene MeO 6 00C, r.t. 8 4 OCl3 N HO NH 3 NaHCO3 CH2Cl2, r.t. N 1 O NaH 2 N N HBr HO reflux 5 7 O Cl3O N OH i.MeMgI,Et2O MeO reflux H ii.HBr,benzene 2 Cl O 1 N Cl N O N DTTA resolve O N O O (TM) recemic product Scheme: 1 Reaction of 3-Methoxy benzaldehyde 8 with MeMgI in ether and subsequent acidification forms 1-(3methoxyphenyl) ethanol 7.Condensation of this alcohol with dimethyl amine 6 produces 1-(3-methoxyphenyl)-N, Ndimethyl ethanamine 5. Demethylation of methoxy group of 5 by refluxing with HBr affords 3-(1(dimethylamino)ethyl)phenole 2(Stedman's procedure)[1].N-ethyl-N-methyl carbamoyl chloride 1, formed from triphosgene 4 and N-ethyl-N-methyl amine 3 in presence of NaHCO3/CH2Cl2 condenses with aminophenol 2 to produce the a racemic product, which on resolution using di-p-toluoyl-D-tartrate (DTTA) affords the required target molecule (T M). 3173 Chittaranjan Bhanja et al J. Chem. Pharm. Res., 2012, 4(6):3171-3183 ______________________________________________________________________________ Retrosynthetic Analysis-2 N N O C-N O N H N + reductive amination O 9 O 1 O 10 O H2N FGI Cl + HO N O-carbamoylation O 6 TM O C-O O O O2N FGI FGA reduction diazotisation nitration 12 11 13 Synthesis-2 O O O O2N Con.HNO3 Con.H2SO4 MeOH Cl 1 O K2CO3 acetone O N O O i.NaNO2/HCl 00C O HO ii.H+/H2O 11 12 13 N H2N H2,Pd/C 10 N N + 6 H ,cat.H NaCNBH3 N O i.L-Tartatic acid O recemic product 9 N ii.NaOH,H2O N O (TM) O Scheme: 2 Nitration reaction of acetophenone 13 with Con.HNO3/H2SO4 forms 3-nitro acetophenone 12.It is then reduced, diazotized and subsequent hydrolysis produces 3-hydroxy acetophenone 10.O-Carbamoylation of 10 with N-ethylN-methylcarbamoyl chloride 1 in presence of K2CO3 affords 9.Reductive amination of 9 with dimethyl amine 6 gives racemic mixture of the product which is then resolved with L(+)-tartaric acid to form the target molecule (TM). Retrosynthetic Analysis-3 N N O N C-O O N O-carbamoylation TM Cl HO + 2 O C-N N H reductive amination 15 6 H2N FGI diazotisation O 1 N O2N N FGI O2N + 12 Synthesis-3 3174 reduction 14 Chittaranjan Bhanja et al J. Chem. Pharm. Res., 2012, 4(6):3171-3183 ______________________________________________________________________________ O N N ,MeOH O N 2 H6 Ti(OiPr)4 , r.t. NaBH4 O2N 12 N CH3OH,r.t. 15 14 N . CAS N HO D-10-CSA NaNO2/ H+ H2N H2/Rany-Ni HO N N HO Na2CO3 H2O-EtOAc C2H5OAc/ C2H5OH 2 2 Cl 1 O K2CO3 L-(+)-Tartaric acid acetone, reflux 2 N N O (TM) O Scheme: 3 The reductive amination of 3-nitroacetophenone 12 with dimethylamine 6 in presence of Ti (OiPr) 4 and NaBH4 in methanol affords 3-(1-(dimethylamino) ethyl) nitrobenzene 15. Hydrogenation of 15 using Raney-Ni produces 3-(1(dimethylamino) ethyl) amino benzene 14.Diazotization of 14 with NaNO2/ H2SO4 gives the racemic 3-(1(dimethylamino) ethyl) phenole 2. The compound 2 is then converted to its CSA salt using D-10-camphorsulfonic acid which produces the same free chiral aminophenol 2 on treatment with Na2CO3. Condensation of 2 with Nethyl-N-methyl carbamoyl chloride (EMCC) 1 affords the target molecule (TM) in the forms of its tartarate salt on subsequent treatment with L+ tartaric acid. Retrosynthetic Analysis:-4 N N N O N C-O O TM O-carbamoylation HO Cl O 1 N FGI 2 H H 16 MeO + 17 2 X C-N + de-methylation 5 2 reductive amination O P Ph N Ph NH2 O MeO Me MeO FGI Zn + Me enantioselective 18 Nu-addition Synthesis:-4 3175 H 19 O MeO H 8 Chittaranjan Bhanja et al J. Chem. Pharm. Res., 2012, 4(6):3171-3183 ______________________________________________________________________________ O MeO MeO [Ph2P(O)NSO] H Kresze rectn. 19 8 O NH2 .HCl 17 O P Ph HN Ph N N 2 MeO H 16 H i.Cat. H+ ii.NaCNBH3 reductive amination MeO HCl/H2O Me Me O P Ph P P N Ph O (BozPHOS) MeO H Me Me i.Me2Zn 18 ii.Cu(OTf)2, toluene,r.t. BBr3 de-methylation HO 2 5 N N Cl N O 1 O O K2CO3 acetone O-carbamoylation (TM) Scheme: 4 Reaction of 3-Methoxy benzaldehyde 8 with p, p-diphenyl N-sulfinylphosphoramidate [Ph2P(O)NOS] forms Ndiphenylphosphinoylimine 19.Copper catalyzed reaction of dimethyl zinc 18 with 19 in presence of bis (phosphine) monoxide chiral ligand affords N-phosphinoylimine as an intermediate. Acid hydrolysis of the intermediate forms amine 17 which on subsequent reductive amination forms 3-methoxy (1-methyl, N, N,-dimethyl) benzyl amine 5.De-methylation of 5 and subsequent reaction with ethylmethylcarbamoyl chloride 1 in presence of a base forms the target molecule (TM). Retrosynthetic Analysis:-5 N N N NH2 C-N HO O O TM OH oximination reduction methylation O FGI HO FGI HO FGI HO C-O de-amination 2 & de-carbonylation N 21 20 10 Synthesis-5 O HO N NH2OH HO OH NH2 LiAlH4 HO H 16 H HCO2H HCl 10 21 20 3176 O N HO 2 Chittaranjan Bhanja et al J. Chem. Pharm. Res., 2012, 4(6):3171-3183 ______________________________________________________________________________ N i. CDI ii. 3 N O O NH O N N O (TM) O resolve N CDI : N N N recemic product Scheme: 5 3-Hydroxy acetophenone 10 is converted to corresponding oxime 21.The oxime is then reduced with LiAlH4 to form 3-(1-aminoethyl) phenol 20.Methylation of the amino group of 20 by reaction with formaldehyde 16 in the presence of formic acid affords 3-(1-(dimethylamino)ethyl)phenole 2.Reaction of 2 with N-methyl ethylamine 3 in presence of carbonyl diimidazole (CDI) inserts a carbonyl group between phenolic oxygen and the N-atom of ethyl methyl amine to form recemic product which on resolution affords the target molecule (TM). Retrosynthetic Analysis:-6 N N N O N C-O N HO Cl FGI MeO FGI + O O 1 O-carbamoylation TM FGI OAc FGI MeO MeO OH MeO FGI enantioselective O-acylation 22 7 17 reductive amination 5 OH NH2 MeO de-methylation 2 7 O MeO FGI reduction 23 Synthesis:-6 OH O NaBH4 MeOH MeO MeO OAc vinyl acetate MeO CAL-B 0 TBME,30 C K2CO3 MeOH-H2O, r.t. (R) 7 23 OH MeO (R) 7 22 i.Phthalimide, PPh3 MeO DEAD, THF, r.t. ii.N2H4 (S) H2O,THF-EtOH,600C 17 3177 N NH2 HCHO, NaBH(OAc)3 Na2SO4,r.t. MeO (S) 5 Chittaranjan Bhanja et al J. Chem. Pharm. Res., 2012, 4(6):3171-3183 ______________________________________________________________________________ N HBr,1000C N HO (S) Cl N N 1 O NaH CH2Cl2,r.t. 2 O (TM) O Scheme: 6 3-Methoxy acetophenone 23 is reduced to 1-(3-methoxyphenyl) ethanol 7 using NaBH4.Enantiomeric esterification of 7 with vinyl acetate forms 22 via Enzymatic Kinetic Resolution using CAL-B(Candida Antarctica Lipase-B) in tetra butyl methyl ether (TBME) solvent. Alkali hydrolysis of 22 produces the corresponding (S) alcohol 7 without the loss of optical purity. Under Mitsunobu reaction condition the alcohol 7 forms 1-(3-methoxyphenyl) ethanamine 17.Dimethylation of the amino group with aq.HCHO 16 followed by addition of Na2SO4 and sod.triacetoxyborohydride (NaBH (OAc) 3) affords 5. Demethylation of 5 with aq.HBr provides 3-(1(dimethylamino) ethyl) phenol 2 .Reaction of phenol 2 with N-ethyl-N-methyl carbamoyl chloride 1 in presence of NaH affords the target molecule (TM). Retrosynthetic Analysis:-7 N N NH O O C-N H O H + N N O hydrogenolysis 24 N Ph O O-carbamoylation 25 Ph O + H O 1 HN Ph N Ph MeO H + 16 27 26 Ph O MeO HO Cl N O 16 TM N N 28 O diastereoselective reductive amination Ph 29 MeO + NH2 23 Synthesis:-7 O HN Ph MeO 23 NH2 MeO 29 i.Ti(OiPr)4,EtOAc ii.H2,Raney-Ni HCl, r.t. Ph O H 16 H N MeO Ph HBr HO HCO2H 28 27 3178 26 Chittaranjan Bhanja et al J. Chem. Pharm. Res., 2012, 4(6):3171-3183 ______________________________________________________________________________ N Cl N N O 1 O i.NaH,THF,r.t. O O NH Ph Pd/C,H2 N r.t. O N N H 16 H,HCO2H L-tartaric acid O O (TM) O 24 25 Scheme: 7 Diastereoselective reductive amination of 3-methoxyacetophenone 23 with (S)-1-phenylethylamine 29 in presence of titanium (IV) isopropoxide and Raney-Ni produces diastereomerically pure amine 28. Methylation of this amine with formaldehyde and formic acid forms 27. Demethylation of ether group of 27 with aq.HBr provides phenol 26.O-Carbamoylation of phenol 26 with carbamoyl chloride 1 affords carbamate 25 and the α-methyl benzyl group from the carbamate is removed by hydrogenolysis to form 24.Second methylation of 24 following the above procedure affords the target molecule (TM) in the form of tartarate salt when treated with L (+)-tartaric acid. Retrosynthetic Analysis:-8 N N O N O amination N FGI O (R) TM O O 30 OH N OAc OH FGI O FGI (R) enantioselective O-acylation 31 O O N FGI O C-O O reduction O O Cl + O 1 O-carbamoylation 9 32 N HO 10 Synthesis:-8 O N HO 10 Polymer-supported Ru-catalyst (I) Novozyme-435 isopropenyl acetate K2CO3 tolune N N O O OH O Cl N 1 O NaH CH2Cl2 O N NaBH4,MeOH O O O 00C 9 32 OH OAc N O O K2CO3 MeOH/H2O (R) 31 N O O (R) ii.Me2NH/THF 30 O I: (TM) polystyrene Scheme: 8 3179 i.MeSO2Cl,Et3N CH2Cl2 Ph Ph O Ph Ru Cl O Ph OC CO Chittaranjan Bhanja et al J. Chem. Pharm. Res., 2012, 4(6):3171-3183 ______________________________________________________________________________ O-Carbamoylation of 3-hydroxyacetophenone 10 with N-ethyl-N-methyl carbamoyl chloride 1 in presence of NaH affords 9, which on reduction with NaBH4 gives 32. Enantiomeric esterification of 32 with isopropenyl acetate forms 31 via Enzymatic Kinetic Resolution using Novozyme-435 and Ru-catalyst (I) in toluene solvent. Alkali hydrolysis of 31 forms the alcohol 30 without the loss of optical purity. Transformation of 30 via a mesylated intermediate affords the target molecule (TM). Retrosynthetic Analysis:-9 N N OAc O N FGI O enantioselective O-acylation TM O O OH FGI 31 32 O O N C-O O O O (R) O N N FGI O-carbomoylation 9 HO Cl + O 10 1 Synthesis:-9 O N HO 1 O K2CO3 ,acetone reflux 10 N N (x) COCl2 N N O N H N N O N O O O 32 9 N Me2NH O OH NaBH4,MeOH 00C OAc vinyl acetate CAL-B N H O Cl 0 10-20 C N O O (TM) (R) 31 Scheme: 9 The reaction of 3-hydroxyacetophenone 10 with N-ethyl-N-methylcarbamoyl chloride 1 in presence of K2CO3 produces 9. Reduction of 9 with NaBH4 affords the recemic alcohol 32. Enantiomeric esterification of 32 with vinyl acetate forms 31 via Enzymatic Kinetic Resolution using CAL-B and bis (heteroarylmethylene) ethane-1, 2diamines (x) in phosgene. The enantiopure acetate (R)-31 on treatment with excess of dimethylamine in toluene affords the desired target molecule (TM). 3180 Chittaranjan Bhanja et al J. Chem. Pharm. Res., 2012, 4(6):3171-3183 ______________________________________________________________________________ Retrosynthetic Analysis-10 N N N O O N reductive amination O desulfinylation TM O O-carbamoylation 34 O S N FGI HO Cl C-O O 33 HN N HN NH2 O O S HO O S FGI + O 1 O S 35 O HO NH2 + 10 37 36 Synthesis-10 O S O HO NH2 37 N O S HN HO NaBH4 O S N HO 1 O K2CO3 (S)-isomer 10 36 HN N O alkali O 35 O S dry HCl/MeOH Cl NH2 N H 16 H Cat H+ O O 34 O N N O O 33 Scheme: 10 Reaction of 3-hydroxy acetophenone 10 with (S) t-butyl sulphinamide 37 first forms N-sulfinylimine 36, which on reduction with NaBH4 forms its corresponding N-sulfinylamine 35. The amine forms its amido-ester 34 with Nethyl-N- methyl carbamoyl chloride 1. Hydrolysis of sulfinyl group of 34 with dry methanolic hydrogen chloride subsequent alkali treatment gives 33.Finally N, N-dimethylation of amine 33 with formic acid and formaldehyde solution furnishes the target molecule (TM). CONCLUSION The synthesis of a particular compound from commercially available starting materials is fundamental to nearly all aspects of organic chemistry.Retrosynthetic analysis/synthon approach is expected to provide new and innovative synthetic strategies in a logical manner for design, execution and development of new synthesis or effect improvements in existing processes. It is a paper exercise; a full analysis of this type will provide many routes for synthesising the target molecule. Exploiting this approach, we have outlined some theoretical propositions in the planning of synthesis of a potent Alzheimer’s disease drug ‘Rivastigmine’.Scalable synthetic routes for newly discovered drug molecules/drug intermediate, useful compounds not available in adequate quantities from natural resources and even the target molecules that have never been synthesized earlier can be best provide by this approach. With the manifestation of new reagents, enantiopure intermediates, chemical reactions and sophisticated 3181 (TM) Chittaranjan Bhanja et al J. Chem. Pharm. Res., 2012, 4(6):3171-3183 ______________________________________________________________________________ new methods of laboratory implementation, it is now time to rethink the synthesis of best selling drugs for market development through this approach. Acknowledgements: The author CB thanks UGC, New Delhi, India for financial support as Teacher Fellow grants. The author also thanks the authorities of IIT Bhubaneswar, IMMT Bhubaneswar and NISER, Bhubaneswar for permission to collect information from books and journals from their library. REFERENCES [1] EJ Corey.Chem.Soc.Rev. 1988, 17,111-133. [2] EJ Corey. Angew. Chem. Int. Ed. Engl. 1999, 30 (5), 455–465. [3] American Psychiatric Association, Am. J. Psychiatry. 1997, 154 (Suppl.) 1–39. [4] NC Berchtold.; CW Cotman.Neurobiol. Aging. 1998 , 19 (3),173-189. [5] RT Bartus.; RL Dean.; B Beer.; AS Lippa. Science. 1982, 217,408–414. [6] EK Perry.; BE Tomlinson.; G Blessed.; K Bergmann.; PH Gibson.; RH Perry. Br. Med. J. 1978, 2, 1457–1459. [7] PJ Whitehouse.; DL Price.; AW Clark.; JT Coyle.; MR DeLong. Ann. Neurol. 1981, 10, 122–126. [8] J Corey-Bloom.; R Anand.; J Veach.Int. J. Geri. Psychopharmacol. 1998, 1, (2), 55-65. [9] M Rosler.; R Anand.; A Cicin-Sain.; S Gauthier.; Y Agid.; P Dal-Bianco.; H B Stähelin.; R Hartman.;M Gharabawi. Br. Med. J. 1999, 318, (7184), 633-640. [10] S I Finkel. Clinical Therapeutics. 2004, 26(7), 980-990. [11] M Rosler.; W Retz.; P Retz-Junginger.; H J Dennler. Behav. Neurol. 1998, 11, (4), 211-216. [12] M Emre.; D Aarsland.; A Albanese. N. Engl. J. Med. 2004, 315, 2509-2518. [13] MR Farlow.; JL Cummings. Am. J. Med. 2007,120, 388-397. [14] PT.Francis.; EK Perry. Brain Cholinergic Syst. Health Dis. 2006, 59-74. [15] http://www.globalbusinessinsights.com/content/rbhc0043t.pdf [16] E Stedman.; E Stedman. J. Chem. Soc. 1929, 609-617. [17] M Weinstock.; M Razin.; M Chorev.; Z Tashma. Adv. Behav. Biol. 1986, 29,539-549. [18] RM Weinstock.; M Chorev.; Z Tashma. EP 193926,1986. [19] MSM Jaweed.; BK Upadhye.; VC Rai.; H Zia. WO 2007026373, 2007. [20] A Enz. GB Patent 2203040, 1987. [21] G Abhay.; M Mangesh.; PR Sanjay. WO2005061446, 2005. [22] S Hana.; H Josef.; S Stanislav. WO2004037771, 2004. [23] DW Ma.; QB Pan.; S Pan. WO2007025481, 2007. [24] M Garrido.; J Vicente.; A Montserrat.; JMJ Miquel. WO2007014973, 2007. [25] AA Boeaio.; J Pytkowicz.; A Cote.; AB Charette. J. Am. Chem. Soc. 2003, 125, 14260; [26] C Lauzon.; J-N Desrosiers.; AB Charette. J.Org.Chem.2005, 70 (25), 10579–10580. [27] M-J Kim.; W-H Kim.; K. Han.; YK Choi.; J Park.J. Org. Chem. 2007, 74, 5304–5310. [28]J Feng.; W-M Chen.; P-H Sun. Jr. of Southern Medical University. 2007, 27 (2), 177-180. [29] J Mangas-Sánchez.; M Rodríguez-Mata.; E.,Busto.; V.Gotor-Fernández.; V. Gotor. J. Org. Chem.2009, 74(15), 5304-5310. [30] M Hu.; F-L Zhang.; M-H Xie.Lett.Org.Chem.2007,4,126-128. [31] M Hu.; F-L Zhang.; M-H Xie. Synth. Commun. 2009, 39, 1527-1533. [32] K Han.; C Kim.; J Park.; M-J Kim. J.Org.Chem. 2010, 75, 3105-3108. [33] K Arunkumar., M Appi Reddy.; T Sravan Kumar.; B Vijaya Kumar.; KB Chandrasekhar.; P Rajender Kumar.; M Pal.Beilstein J. Org. Chem. 2010, 6, 1174–1179. [34]VR Arava1.; L Gorentla.; PK Dubey. Int J.Org. Chem. 2011, 1, 26-32. [35] A Fisher. “Advances in Alzheimer's and Parkinson's Disease: Insights, Progress, and Perspectives”. Springer, New York, 2008, pp-4. [36] J J Buccafusco. “Cognitive Enhancing Drugs” Birkhauser -Vergla, Baseal, Switzerland, 2004, pp-188. [37]E J Corey, B Czakó, L Kürti. “Molecules and Medicine” John Wiley & Sons, Inc, New Jersey, 2007, pp-225. [38] WO Foye, TL Lemke. “Foye’s Principles of Medicinal Chemistry” Lippinicott Williams & Wilkins, Philadelphia, 2008, pp-377. [39] E J Corey; X M Chang. “The Logic of Chemical Synthesis” John Wiley & Sons, New York, 1989. [40] S Warren. “Organic Synthesis-The Disconnection Approach”. John Wiley & Sons, 1982. 3182 Chittaranjan Bhanja et al J. Chem. Pharm. Res., 2012, 4(6):3171-3183 ______________________________________________________________________________ [41] J Clayden, N Greeves, S Warren, P Wothers. “Retrosynthetic Analysis in Organic Chemistry” Oxford University Press Inc., New York, 2001; pp. 773-778. 3183