Experiment Synthesis of t
advertisement
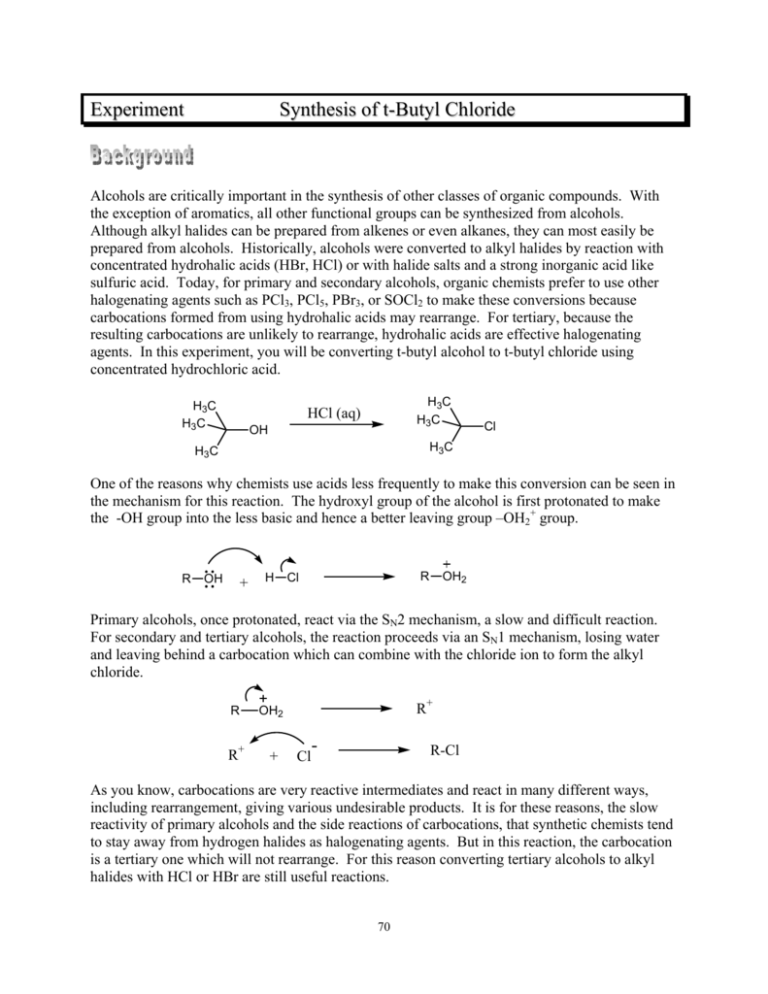
Experiment Synthesis of t-Butyl Chloride Alcohols are critically important in the synthesis of other classes of organic compounds. With the exception of aromatics, all other functional groups can be synthesized from alcohols. Although alkyl halides can be prepared from alkenes or even alkanes, they can most easily be prepared from alcohols. Historically, alcohols were converted to alkyl halides by reaction with concentrated hydrohalic acids (HBr, HCl) or with halide salts and a strong inorganic acid like sulfuric acid. Today, for primary and secondary alcohols, organic chemists prefer to use other halogenating agents such as PCl3, PCl5, PBr3, or SOCl2 to make these conversions because carbocations formed from using hydrohalic acids may rearrange. For tertiary, because the resulting carbocations are unlikely to rearrange, hydrohalic acids are effective halogenating agents. In this experiment, you will be converting t-butyl alcohol to t-butyl chloride using concentrated hydrochloric acid. H3C H3C H3C H3C HCl (aq) OH Cl H3C H3C One of the reasons why chemists use acids less frequently to make this conversion can be seen in the mechanism for this reaction. The hydroxyl group of the alcohol is first protonated to make the -OH group into the less basic and hence a better leaving group –OH2+ group. R OH + H R Cl OH2 Primary alcohols, once protonated, react via the SN2 mechanism, a slow and difficult reaction. For secondary and tertiary alcohols, the reaction proceeds via an SN1 mechanism, losing water and leaving behind a carbocation which can combine with the chloride ion to form the alkyl chloride. R OH2 R+ + R+ Cl - R-Cl As you know, carbocations are very reactive intermediates and react in many different ways, including rearrangement, giving various undesirable products. It is for these reasons, the slow reactivity of primary alcohols and the side reactions of carbocations, that synthetic chemists tend to stay away from hydrogen halides as halogenating agents. But in this reaction, the carbocation is a tertiary one which will not rearrange. For this reason converting tertiary alcohols to alkyl halides with HCl or HBr are still useful reactions. 70 Pre-lab Preparation Before coming to lab, you must complete the following assignment. 1. Review the material on extraction and washing in your techniques textbook. 2. In your notebook, write the relevant physical constants for t-butyl alcohol and t-butyl chloride (boiling point, density, solubility in water). 3. In your notebook, explain the purpose of : a. washing the organic layer with sodium bicarbonate solution. b. washing the organic layer with water. c. treating the organic layer with anhydrous calcium chloride. 4. What boiling point range should you collect to obtain a reasonably pure sample of the correct product? Experimental Procedure ! Safety Considerations ! Be careful with handling concentrated (12M) hydrochloric acid. Measure it in the hood. It gives off harmful HCl gas. ! In the sodium bicarbonate wash purification step, CO2 gas is evolved. Avoid pressure buildups in the separatory funnel by venting often. Note: The reaction of t-butyl chloride in aqueous HCl is a rapid one. This reaction is performed in a separatory funnel because shaking the funnel allows the two immiscible phases to mix in order for the reaction to proceed. Into a 50-mL separatory funnel, add 8.0 g of t-butyl alcohol, and 25 mL of concentrated (12M) hydrochloric acid. Without putting a stopper in the separatory funnel, swirl the funnel for two or three minutes to thoroughly mix the reactants. Then, stopper the funnel and shake the contents for approximately five minutes, venting the funnel often during the process to relieve any buildup of pressure. By the end of this time, the reaction will be completed. In the purification steps that follow, keep in mind that the alkyl chloride product has a boiling point that is very low. Therefore, you must be concerned about evaporation of your product. Whenever possible, keep containers of your product tightly stoppered. 71 Place the separatory funnel onto a ring stand and let the reaction mixture sit until the two layers have separated completely. Remove the aqueous layer, making certain to keep the organic layer, the one containing your product. Wash the organic layer in your separatory funnel with two 20mL portions of 5% sodium bicarbonate solution. Note: You should know what “washing” means. After washing the organic layer once with sodium bicarbonate solution, separate the layers. Add the second bicarbonate wash to the organic layer and separate the layers again. Once you are sure that you have the organic layer, discard the aqueous bicarbonate layers. This is what is meant by “…wash with two portions of aqueous sodium bicarbonate solution.” Vent your separatory funnel early and often during these washing steps since gas will be evolved during the process. Then wash the organic layer with a 15-mL portion of water. Pour the organic layer into a small Erlenmeyer flask and add enough calcium chloride pellets so the just- added pellets don’t clump to one another. Stopper the flask for ten minutes while you set up a distillation apparatus. Set up a simple distillation flask using a 15-mL round bottom flask as the distillation flask. Make sure that all parts of the apparatus are completely dry. Decant the product, leaving the CaCl2 pellets behind, into the 15-mL round bottom, add boiling stones and begin to distill to purify your product. Collect only material that you believe to be the correct product in a tared 15-mL round bottom flask. Always record the boiling point range observed. Weigh your flask with its contents to determine the yield of t-butyl chloride. Your instructor may ask you to run an IR of your product. Post-Lab and Report Requirements 1. A balanced equation for the reaction 2. A detailed mechanism for the reaction 3. Yield information a. determination of the limiting reagent in the reaction b. determination of the theoretical yield in grams of product formed c. determination of percentage yield 4. Give the chemical reasons why you did not achieve a 100% yield. 5. Your yield loss analysis above should have included the formation, from t-butyl alcohol, of another liquid product which would have reduced the yield of your t-butyl chloride product. Give a detailed mechanism for the formation of this product. Explain why more of this product doesn’t form. 6. If an IR was run, analyze the spectrum. Does it prove that the reaction occurred as expected? 72