CHAPTER 4 ATOMIC STRUCTURE AND BONDING
CHAPTER 4 SOLIDIFICATION, CRYSTALLINE IMPERFECTIONS, AND DIFFUSION IN SOLIDS
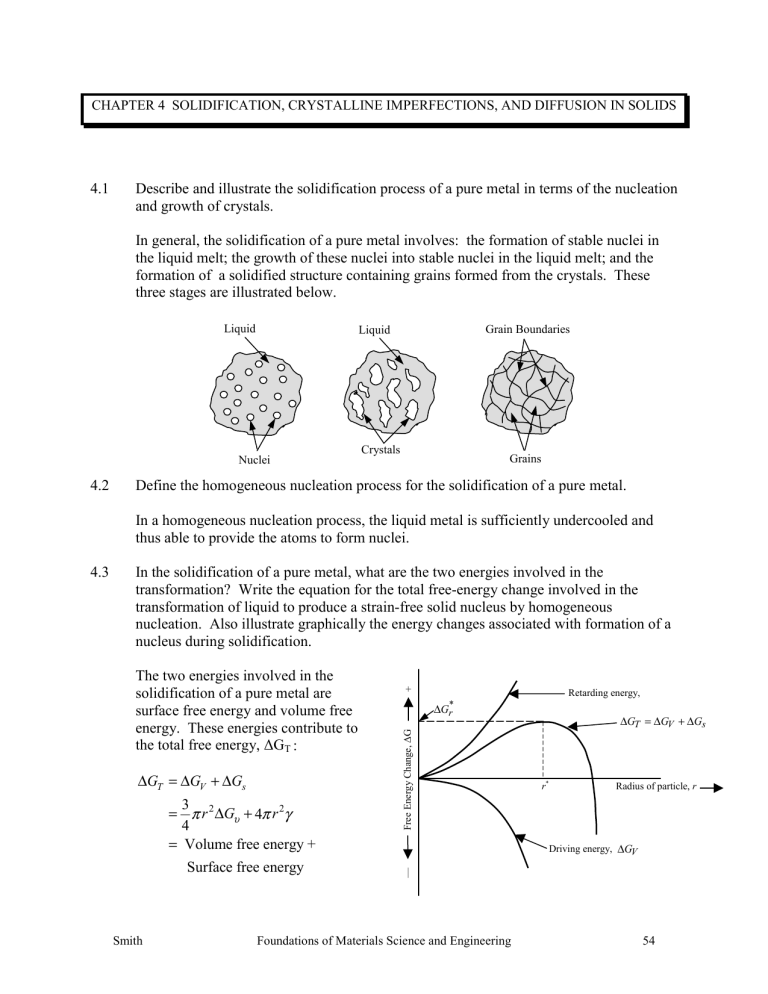
4.1 Describe and illustrate the solidification process of a pure metal in terms of the nucleation and growth of crystals.
In general, the solidification of a pure metal involves: the formation of stable nuclei in the liquid melt; the growth of these nuclei into stable nuclei in the liquid melt; and the formation of a solidified structure containing grains formed from the crystals. These three stages are illustrated below.
Liquid Liquid Grain Boundaries
Crystals
Nuclei Grains
4.2 Define the homogeneous nucleation process for the solidification of a pure metal.
In a homogeneous nucleation process, the liquid metal is sufficiently undercooled and thus able to provide the atoms to form nuclei.
4.3 In the solidification of a pure metal, what are the two energies involved in the transformation? Write the equation for the total free-energy change involved in the transformation of liquid to produce a strain-free solid nucleus by homogeneous nucleation. Also illustrate graphically the energy changes associated with formation of a nucleus during solidification.
The two energies involved in the solidification of a pure metal are +
Retarding energy,
∆ G
* r surface free energy and volume free energy. These energies contribute to the total free energy, ∆ G
T
:
∆ G
T
= ∆ G
V
+ ∆ G s
∆ G
T
= ∆ G
V
+ ∆ G s r * Radius of particle, r
=
=
3
4
π ∆
υ
+ 4 r
Volume free energy +
Surface free energy
Driving energy, ∆ G
V
Smith Foundations of Materials Science and Engineering 54
4.4 In the solidification of a metal, what is the difference between an embryo and a nucleus?
What is the critical radius of a solidifying particle?
An embryo refers to a solidified cluster of atoms which is unstable and can thus redissolve. A nucleus is a comparatively larger stable cluster which can grow into a crystal. The dimension defining the boundary between an embryo and a nucleus is the critical radius.
4.5 During solidification, how does the degree of undercooling affect the critical nucleus size? Assume homogeneous nucleation.
In general, the greater the degree of undercooling of a liquid melt, the smaller the critical radius of the nuclei formed.
4.6 Distinguish between homogeneous and heterogeneous nucleation for the solidification of a pure metal.
In homogeneous nucleation of a liquid metal, the nuclei are formed from atoms of the metal solidifying. In heterogeneous nucleation, impurity atoms or container surfaces act as nucleating agents.
4.7 Calculate the size (radius) of the critically sized nucleus for pure platinum when homogeneous nucleation takes place.
The critical radius of nucleus is calculated as, r * =
∆
− 2 γ
H f
T
∆ m
T where γ = × − 7
0.2
∆
T m
H
= f
= − T m
= 2045 K
Substituting, and the undercooling r * =
− × − 7
=
4.8 Calculate the number of atoms in a critically sized nucleus for the homogeneous nucleation of pure platinum.
The number of atoms in a critically sized nucleus is found as,
Volume of nucleus
Volume per atom
=
Volume of nucleus
(Vol. of unit cell)(No. atoms per unit cell)
Using the critical radius result of Problem 4.7,
Smith Foundations of Materials Science and Engineering 55
Vol. of critical-sized nucleus =
Vol. of unit cell of Pt = a 3 =
4
3
π r *3 =
4
3
π × − 7 3 = × − 21 cm 3
× − 9 3 = × − 29 m 3 = × − 23 cm 3
Vol. per atom =
Substituting,
3.574 10 − 23 cm 3
4 atoms/FCC unit cell
= 8.9
× − 24 cm /atom
Volume of nucleus
×
× 3
Volume per atom
=
8.935 10 cm /atom
= 641 atoms
4.9 Calculate the size (radius) of the critical nucleus for pure iron when nucleation takes place homogeneously.
For iron, γ = × − 7 ∆ H f
= − T m
= 1808 K
The amount of undercooling is then 0.2
T m
= the critical radius becomes, r * =
− 2 γ T m
∆ H f
∆ T
=
−
−
× − 7
= 9.72×10 cm
4.10 Calculate the number of atoms in a critically sized nucleus for the homogeneous nucleation of pure iron.
The relevant volumes, based on the solution of Problem 4.9, are:
Vol. of critical-sized nucleus =
4
3
π r *3 =
4
3
π × − 8 3 = × − 21 cm 3
Vol. of unit cell of Pt = a 3 = × − 9 3 = × − 29 m 3 = × − 23 cm 3
Vol. per atom =
2.355 10
− 23 cm 3
2 atoms/BCC unit cell
= 1.1
× − 23 cm /atom
Thus, the number of atoms in a critically sized nucleus is:
Volume of nucleus
×
× 3
Volume per atom
=
1.178 10 cm /atom
= 327 atoms
4.11 Describe the grain structure of a metal ingot that was produced by slow-cooling the metal in a stationary open mold.
In general, equiaxed grains are formed adjacent to the cold mold wall where rapid
Smith Foundations of Materials Science and Engineering 56
cooling occurs during solidification. Elongated columnar grains are formed in the metal ingot interior, in the direction of thermal gradients, due to slow cooling of the metal in the mold interior.
4.12 Distinguish between equiaxed and columnar grains in a solidified metal structure.
Equiaxed grain dimensions are approximately equal in all directions whereas columnar grains are elongated in a particular direction.
4.13 How can the grain size of a cast ingot be refined? How is grain refining accomplished industrially for aluminum alloy ingots?
The grain size of a cast ingot can be refined by: solidifying the metal at a rapid rate; and adding grain refining agents (heterogeneous nucleating agents). Grain refining of aluminum ingots is accomplished through chill casting and by adding grain refining agents such as titanium and/or boron.
4.14 What special techniques must used to produce single crystals?
Single crystals can be produced by introducing a single crystal as a seed crystal. The seed continuously rotates as it is slowly lowered and then withdrawn from the melt.
4.15 How are large silicon single crystals for the semiconductor industry produced?
Large single crystals of silicon are produced using a pure silicon seed crystal with a pure silicon melt (Czochralski process).
4.16 What is a metal alloy? What is a solid solution?
A metal alloy is a mixture of two or more metals or of a metal (metals) and a non-metal
(nonmetals). A solid solution is a type of alloy which is solid and consists of two or more elements atomically dispersed in a single phase structure.
4.17 Distinguish between a substitutional solid solution and an interstitial solid solution.
A substitutional solid solution is one in which the solute atoms of the elements replace those of the solvent atoms in the crystal lattice. An interstitial solid solution is one in which the solute atoms of the elements are positioned in the interstitial spaces between the solvent atoms of the crystal lattice.
4.18 What are the conditions that are favorable for extensive solid solubility of one element in another?
Four conditions favor extensive solid solubility:
1. less than 15% difference between the atomic diameters of the elements forming the solid solution;
Smith Foundations of Materials Science and Engineering 57
2. identical valence of the elements;
3. similar electronegativities;
4. common crystal structure of the elements.
4.19 Using the data in the following table, predict the relative degree of solid solubility of the following elements in aluminum:
(a) copper (c) magnesium (e) silicon
(b) manganese (d) zinc
Use the scale very high, 70–100%; high, 30–70%; moderate, 10–30%;low, 1–10%; and very low, < 1%.
Element Atom Radius
(nm)
Crystal
Structure
Electronegativity
Valence
Aluminum 0.143 FCC 1.5 +3
Manganese 0.112 Cubic 1.6 +2, +3, +6, +7
Magnesium 0.160 HCP 1.3 +2
Diamond 1.8 +4
(a) low (b) (c) (d) low
4.20 Using the data in the following table, predict the relative degree of atomic solid solubility of the following elements in iron:
(c) molybdenum (e) manganese
(d) titanium
(a) nickel
(b) chromium
Use the scale very high, 70–100%; high, 30–70%; moderate, 10–30%;low, 1–10%; and very low, < 1%.
Element Atom Radius
(nm)
Crystal
Structure
Electronegativity
Valence
BCC
Nickel 0.125
Chromium 0.125 BCC 1.6
Molybdenum
Titanium
Manganese
0.136
0.147
0.112
BCC
HCP
Cubic
1.3
1.3
1.6
+2, +3, +6
+3, +4, +6
+2, +3, +4
+2, +3, +6, +7
(a) high (b) (c) (d) moderate
Smith Foundations of Materials Science and Engineering 58
4.21 Calculate the radius of the largest interstitial void in the BCC α iron lattice. The atomic radius of the iron atom in this lattice is 0.124 nm, and the largest interstitial voids occur at the (¼, ½, 0); (½, ¾, 0); (¾, ½, 0); (½, ¼, 0), etc., type positions.
For BCC crystal structure, a =
4 R
3
=
4(0.124 nm)
3
= 0.286 nm a
4 x
Letting x = Fe atom radius + Interstitial void radius, a
R
Fe
R void x 2 =
1
16 a 2 +
1
4 a 2 =
5
16 a 2 a x =
5
16 a = 0.559
a = =
2
The interstitial void radius is thus,
R void
= −
Fe
= = 0.036 nm
4.22 Describe and illustrate the following types of point imperfections that can be present in metal lattices: ( a ) vacancy, ( b ) divacancy, and ( c ) interstitialcy. a) A vacancy, a point defect, is an atomic site which is missing an atom. b) A divacancy is a defect in a crystal lattice where two atoms are missing from adjoining atomic sites. c) An interstitialcy is a point defect where an atom occupies an interstitial site between surrounding atoms in normal sites.
Vacancy
Interstitialcy
4.23 Describe and illustrate the following imperfections that can exist in crystal lattices:
(a) Frenkel imperfection and (b) Schottky imperfection. a) A Frenkel imperfection is a vacancy-interstitialcy pair which sometimes occurs in ionic crystals.
Smith Foundations of Materials Science and Engineering 59
b) A Schottky imperfection is a cation-anion divacancy which sometimes occurs in ionic crystals.
Frenkel Imperfection Schottky Imperfection
4.24 Describe and illustrate the edge– and screw–type dislocations. What type of strain fields surround both types of dislocations?
An edge dislocation is a line imperfection caused by an extra half plane of atoms between two normal planes of atoms. Whereas a screw dislocation is a line imperfection created by applying upward and downward shear stress to regions of a perfect crystal separated by a common plane.
Dislocation Line Screw Dislocation
The strain fields associated with the edge and screw dislocations are shown below:
Smith Foundations of Materials Science and Engineering 60
4.25 Describe the structure of a grain boundary. Why are grain boundaries favorable sites for the nucleation and growth of precipitates?
Grain boundaries are surface imperfections that separate grains of different orientations.
The grain boundary, a narrow region between two grains, is approximately two to five atomic diameters in width and contains mismatched atoms from adjacent grains. Grain boundaries are favorable sites for the nucleation and growth of precipitates because the irregular atom arrangement at grain boundaries provides lower atomic packing and high energy. Atoms are thus able to diffuse more rapidly to form precipitates.
4.26 Why are grain boundaries easily observed in the optical microscope?
Grain boundaries can be easily observed under an optical microscope because they etch more rapidly than grains. Chemical etching thus produces tiny grooves along grain boundaries which appear as dark lines under an optical microscope because they reflect light less intensely.
4.27 How is the grain size of polycrystalline materials measured by the ASTM method?
In the ASTM method of measuring grain size of polycrystalline materials, the grain size number, n , is defined by the equation N = 2 n − 1 , where N is the number of grains per square inch, measured on a polished and etched surface at a magnification of 100x.
4.28 If there are 600 grains per square inch on a photomicrograph of a metal at 100x, what is its ASTM grain-size number?
N = n − 1
= − 1)(ln 2)
= − 1)(0.693) n = 9.23 1 10.23
Smith Foundations of Materials Science and Engineering 61
4.29 If there are 400 grains per square inch on a photomicrograph of a ceramic material at
200x, what is its ASTM grain-size number?
N = = n − 1
= − 1)(ln 2)
= − 1)(0.693) n = 10.64 1 11.64
4.30 Determine, by counting, the ASTM grain-size number of the low–carbon sheet steel shown in Fig. P4.30. This micrograph is at 100x.
Estimating 40 grains/in 2 from the micrograph,
N = = n − 1
= − 1)(ln 2)
= − 1)(0.693) n = 5.3 1 6.3
4.31 Determine the ASTM grain-size number of the type 430 stainless steel micrograph shown in Fig. P4.31.This micrograph is at 200x.
Estimating 100 grains/in 2 from the micrograph,
No. of grains at 100 × =
200 2
100 2
(100)
= 400 grains/in 2
N = = n − 1
= − 1)(ln 2) n =
= −
8.64 1
1)(0.693)
9.64
4.32 What is a thermally activated process? What is the activation energy for such a process?
A thermally active process is one which requires a definite amount of thermal energy to overcome an activation energy barrier and enter the reactive state.
4.33 Write an equation for the number of vacancies present in a metal at equilibrium at a particular temperature and define each of the terms. Give the units for each term and use electron volts for the activation energy.
Smith Foundations of Materials Science and Engineering 62
where n
E
N
T v v n v
= NCe
− /
=
=
number of vacancies per cubic meter of metal
total number of atom sites per cubic meter of metal
= activation energy to form a vacancy (eV)
= absolute temperature (K) k = Boltzmann's = × − 6
C = constant
4.34 (a) Calculate the equilibrium concentration of vacancies per cubic meter in pure copper at 850ºC. Assume that the energy of formation of a vacancy in pure copper is 1.00 eV.
(b) What is the vacancy fraction at 800ºC? a) In general, the equilibrium number of vacancies is n v
= NCe
− / . For copper,
6 3
N
N o
ρ
Cu at. mass Cu
=
×
(63.54 g/at. mass)
Substituting and assuming E v
= 1.00 eV at 1123 K, n
υ
=
= × 3
−
×
×
1.00 eV
=
×
= 3 b) The vacancy fraction at 1073 K is, n
N
υ = exp
×
− 1.00 eV
= e
− 10.81
= 2.02×10 vacancies/atom
4.35 (a) Calculate the equilibrium concentration of vacancies per cubic meter in pure silver at
750ºC. Assume that the energy of formation of a vacancy in pure silver is 1.10 eV. (b)
What is the vacancy fraction at 700ºC? a) The equilibrium number of vacancies is calculated as n v
= NCe
− / .
Thus for silver,
N =
N o
ρ
Cu at. mass Cu
=
×
(107.870 g/at. mass)
× 6 3
=
Substituting and assuming E v
= 1.10 eV for vacancies formed at 1023 K,
× 3
3
Smith Foundations of Materials Science and Engineering 63
n
υ
= × 3
−
×
1.10 eV
= b) The vacancy fraction at 973 K is,
3 n
N
υ = exp
×
− 1.10 eV
= e − 13.12
= 2.01×10 vacancies/atom
4.36 Write the Arrhenius rate equation in the (a) exponential and (b) common logarithmic forms. a) Rate of reaction = Ce − Q RT
10
−
Q
2.303
RT
4.37 Draw a typical Arrhenius plot of log
10
of the reaction rate versus reciprocal absolute temperature, and indicate the slope of the plot.
.
A typical Arrhenius plot of the logarithmic reaction rate is shown below for the SI absolute Kelvin temperature scale. The relationship between the log
10
of the reaction rate and the inverse absolute temperature is linear with a slope of – Q /(2.303
R ).
Intercept = log
10
(const.)
Slope = −
=
∆
∆
Q
2.303
R
T, K
1
, K
-1
T
4.38 Describe the substitutional interstitial diffusion mechanisms in solid metals.
Smith Foundations of Materials Science and Engineering 64
During substitutional diffusion of atoms in a solid alloy crystal lattice, solute atoms move into positions of solvent atoms in the matrix through a vacancy mechanism. In interstitial diffusion, small solute atoms move between the interstices of the solvent lattice.
4.39 Write the equation for Fick’s first law of diffusion, and define each of the terms in SI units.
Fick’s first law of diffusion is given by:
J = − D dC dx
or in SI unit form,
atoms m 2 ⋅ s
=
m s
2
m 3
× m
where J = flux or net flow of atoms;
D = proportionality constant called the diffusivity (atomic conductivity) or
diffusion dC
= concentration gradient. dx
4.40 What factors affect the diffusion rate in solid metal crystals?
The diffusion rate in solid metal crystals is affected by five factors:
1. Type of diffusion mechanism;
2. Temperature of diffusion;
3. Concentration of the diffusion species (concentration gradient);
4. Type of crystal structure;
5. Type of crystal imperfections present.
4.41 Write the equation for Fick’s second law of diffusion in solids and define each of the terms.
Fick’s second law of diffusion in solids, written for the x -direction, is: dC dt x = d dx
D dC x dx where dC x dt
= rate of change of the concentration of the diffusing species in the x-direction; dC x
D dx
=
= concentration gradient of the diffusing species in the x-direction;
diffusion coefficient of the diffusing species.
4.42 Write the equation for the solution to Fick’s second law for the diffusion of a gas into the surface of a solid metal crystal lattice.
Smith Foundations of Materials Science and Engineering 65
Fick’s second law of diffusion, for the diffusion of a gas into the surface of a solid metal crystal lattice is:
C s
− C x
C s
− C o
= erf
2 x
Dt
where C s
= surface concentration of element in gas diffusing into the surface;
C o
= initial uniform concentration of element in solid;
C x
= x
D
= distance from surface;
= diffusivity of diffusing solute element; t = time.
4.43 Describe the gas-carburizing process for steel parts. Why is the carburization of steel parts carried out?
In the gas carburizing process for steel parts, the parts are placed in a furnace in contact with a gas rich in CO at about 927ºC. The carbon from the gas diffuses into the surface of the steel part and increases the carbon content of the outer surface region of the part.
The higher carbon concentration at the surface makes the steel harder in this region. A steel part can thus be produced with a hard outer layer and a tough low carbon steel inner core. This duplex structure is important, for example, for many types of gears.
4.44 Consider the gas carburizing of a gear of 1018 steel (0.18 wt %) at 927ºC (1700ºF).
Calculate the time necessary to increase the carbon content to 0.35 wt % at 0.40 mm below the surface of the gear. Assume the carbon content at the surface to be 1.15 wt % and that the nominal carbon content of the steel gear before carburizing is 0.18 wt %. D
(C in λ iron) at 927ºC = 1.28 × 10 –11 m 2 /s.
The time required for this diffusion process is calculated using Fick’s second law,
C s
− C x
C s
− C o
= erf
2 x
Dt
where: C s x =
= 1.15% C o
=
×
0.18% C x
=
D
927
0.35%
C
= 1.28 10 − 11 m / s
Substituting = erf
×
− 4 t
55.90
t
= erf z
Smith Foundations of Materials Science and Engineering 66
Interpolating from Table 4.5, erf z z
0.8209 0.95
0.8247 x
0.8427 1.0
Thus, t z x − 0.95
=
55.90
t
= 0.959
= 3397.7 s = 56.6 min.
x = 0.959
4.45 The surface of a steel gear made of 1022 steel (0.22 wt % C) is to be gas-carburized at
927ºC (1700ºF). Calculate the time necessary to increase the carbon content to 0.30 wt % at 0.030 in. below the surface of the gear. Assume the carbon content of the surface to be
1.20 wt %. D (C in λ iron) at 927ºC = 1.28 × 10 –11 /s.
Given: C s
= 1.20% C o
= 0.22% C x
= 0.30%
m 2 x = 0.03 in.(0.3048 m/ft) ft
12 in.
× D
927 , C
= 1.28 10
− 11 m / s
C s
− C x
C s
− C o
= = erf
×
− 4
2 t
106.49
t
Interpolating from Table 4.5,
= erf z x − 1.2
x = 1.234
erf z z
0.9103 1.2
0.9184 x
0.9340 1.3
Thus, t =
106.49
z
2
=
106.49
1.234
2
= 7, 446.6 s = 124 min.
4.46 A gear made of 1020 steel (0.20 wt % C) is to be gas-carburized at 927ºC (1700ºF).
Calculate the carbon content at 0.90 mm below the surface of the gear after a 4.0-hour carburizing time. Assume the carbon content at the surface of the gear is 1.00 wt %.
D (C in λ iron) at 927ºC = 1.28 × 10 –11 m 2 /s.
Given: C s
= 1.00% x =
C o
= 0.20%
×
C x
= ?
D
927 C t =
=
4 h = 14,400 s
× − 11 m / s
Smith Foundations of Materials Science and Engineering 67
C s
− C x
C s
− C o
=
1.00
− C x = erf
− 4
× 2 x = 0.8608
0.8427 1.00 x 1.0482
0.8802 1.10
Substituting,
1.25(1 − C x
) 0.8608
C x
= 0.311 wt %
4.47 A gear made of 1020 steel (0.20 wt % C) is to be gas-carburized at 927ºC (1700ºF).
Calculate the carbon content at 0.04 in. below the surface of the gear after a 7.0-hour carburizing time. Assume the carbon content at the surface of the gear is 1.15 wt %.
D (C in λ iron) at 927ºC = 1.28 × 10 –11 m 2 /s.
Given: C s x
= 1.15%
=
C o
= 0.20%
×
C x
= ?
D
927 t
C
=
=
7 h = 25,200 s
× − 11 m / s
1.25(1 − C x
) erf (1.0482)
Interpolating from Table 4.5, erf z z
−
= x − 0.8427
−
C
C s s
−
−
C
C x o
=
1.15
− C x = erf
× 2
− 3
1.15
−
0.95
C x =
Interpolating from Table 4.5, erf (0.89798) erf z z
0.7707 0.85 x 0.89798
0.7970 0.90
Substituting,
1.15
−
0.95
C x
=
= 0.7959
x − 0.7707
C x x = 0.7959
= 0.394 wt %
Smith Foundations of Materials Science and Engineering 68
4.48 The surface of a steel gear made of 1018 steel (0.18 wt % C) is to be gas-carburized at
927ºC (1700ºF). Calculate the time necessary to increase the carbon content to 0.35 wt
% at 1.00 mm below the surface. Assume the carbon content of the surface of the gear is
1.20 wt %. D (C in λ iron) at 927ºC = 1.28 × 10 –11 m 2 /s.
Given: C s x
=
=
1.20% C o
= 0.18%
×
C x
=
D
927
0.35%
C
= × − 11 m / s
C s
− C x
C s
− C o
= = erf
×
− 3
2 t
139.75
t
Interpolating from Table 4.5,
= erf z x − 0.95
x = 0.978
erf z z
0.8209 0.95
Thus, t =
139.75
z
2
=
139.75
0.978
2
0.8333 x
0.8427 1.0 t = 20, 400 s = 340 min. = 5.67 h
4.49 A gear made of 1020 steel (0.20 wt % C) is to be gas-carburized at 927ºC (1700ºF).
Calculate the carbon content at 0.95 mm below the surface of the gear after an 8.0–hour carburizing time. Assume the carbon content at the surface of the gear is 1.25 wt %.
D (C in λ iron) at 927ºC = 1.28 × 10 –11 m 2 /s.
Given: C s x
=
=
1.25% C o
= 0.20%
×
C x
= ?
D
927 C t =
=
8 h = 28,800 s
1.28 10 − 11 m / s
C
C s s
−
−
C
C x o
=
1.25
− C x = erf
× 2
− 4
1.25
−
1.05
C x = erf (0.7823)
Smith Foundations of Materials Science and Engineering 69
Interpolating from Table 4.5,
C s t =
C o
7.5 h = 27,000 s D
927
C x
C
=
−
= x − 0.7112
−
× − 11 m / s x = 0.7312
erf z z
0.7112 0.75 x 0.7823
0.7421 0.80
Substituting,
1.25
−
1.05
C x = 0.7312
C x
= 0.48 wt %
4.50 A gear made of 1018 steel (0.18 wt % C) is to be gas-carburized at 927ºC (1700ºF). If the carburizing time is 7.5 h, at what depth in millimeters will the carbon content be 0.40 wt %? Assume the carbon content at the surface of the gear is 1.20 wt %. D (C in λ iron) at 927ºC = 1.28 × 10 –11 m 2 /s.
Given: = 1.20% = 0.18% = 0.40%
C
C s s
−
−
C
C x o
= = erf
× x
2
Interpolating from Table 4.5,
= = z erf z z
0.7707 0.85
0.7843 x
1
0.7970 0.90
−
= x
1
−
−
0.85
0.90 0.85
Substituting, z x
=
= x
0.00103 m = 1.03 mm x
1
= 0.8759
4.51 If boron is diffused into a thick slice of silicon with no previous boron in it at a temperature of 1100ºC for 5 h, what is the depth below the surface at which the concentration is 10 18 atoms/cm 3 ?
D = 4 × 10 –13 cm
17 atoms/cm 3 if the surface concentration is 10
2 /s for boron diffusing in silicon at 1100ºC.
Given: C s
= 3 C x
= 3 C o
= 0.0
t = × D
1100 C
= × − 13 cm / s
Smith Foundations of Materials Science and Engineering 70
C s
− C x
C s
− C o
=
10 18 − 10 17
10 18 − 0
= erf
x
2
× × 4 x
− 4
= erf z
Interpolating from Table 4.5,
C
C s s
−
−
C
C x o
=
10 18 − 10 16
10 18 − 0
= erf x
1
− 1.1
= 1.166
erf z z
0.8802 1.1
0.9000 x
0.9103 1.2
Substituting, z x
=
=
1.166
=
− 4 x
− 4
4.52 If aluminum is diffused into a thick slice of silicon with no previous aluminum in it at a temperature of 1100ºC for 6 h, what is the depth below the surface at which the concentration is 10 16 atoms/cm 3 surface concentration is 10
D = 2 × 10 –12 cm 2
18 atoms/cm 3 ?
/s for aluminum diffusing in silicon at 1100ºC.
if the
Given: C s
= 3 C x
= 3 C o
= 0.0
t = × D
1100 C
= × − 12 cm / s
× x
2 × x
1
4
x
− 4
= erf z
Interpolating from Table 4.5, erf z z
0.9891 1.8
0.9900 x
0.9928 1.9 x
1
− 1.8
Substituting, z x
= 1.824
=
= x
4.157 10 − 4
− 4 x
1
= 1.824
Smith Foundations of Materials Science and Engineering 71
4.53 Phosphorus is diffused into a thick slice of silicon with no previous phosphorus in it at a temperature of 1100ºC. If the surface concentration of the phosphorus is 1 × 10 18 atoms/cm 3 and its concentration at 1 µm is 1 × 10 diffusion time be? D = 3.0 × 10 –13 cm 2
15 atoms/cm 3 , how long must the
/s for P diffusing in Si at 1100ºC.
Given: C s
= 3 C x
= 3 C o
= 0.0
x = µ × D
1100 , C
= × − 13 cm / s
C s
− C x
C s
− C o
=
10 18 − 10 15
10 18 − 0
= erf
ª
«
× 2 t
º
»
91.287
t
Interpolating from Table 4.5,
= erf z x − 2.2
x = 2.35
erf z z
Thus,
0.9981 2.2 t =
ª
¬
91.287
z
º
¼
2
=
ª
¬
91.287
2.35
º
¼
2
0.9990 x
0.9993 2.4 t =
4.54 If the diffusivity in Prob. 4.53 had been 1.5 × 10 –13 cm 2 /s, at what depth in micrometers would the phosphorus concentration be 1 × 10 15 atoms/cm 3 ?
Since
C s
− C x
C s
− C o
=
1508 s = 25.1 min.
z =
ª
« x
º
» = 2.35, x = − 5 0.707 µm
× 2
4.55 Arsenic is diffused into a thick slice of silicon with no previous arsenic in it at 1100ºC. If the surface concentration of the arsenic is 5 × 10
1.2 µm below the silicon surface is 1.5 × 10 time be? ( D = 3.0 × 10 –14 cm 2
16
18 atoms/cm 3
atoms/cm 3
, and its concentration at
, how long must the diffusion
/s for As diffusing in Si at 1100ºC.)
Given: C s
= × 3 C x
= × 3 C o
= 0.0
x = × × -4 D
1100 , C
= × − 14 cm / s
Smith Foundations of Materials Science and Engineering 72
C s
− C x
C s
− C o
=
× 18 − × 16
× 18 − 0
= erf
ª
«
×
− 4
2 t
º
»
§
©
346.4
t
·
¹
Interpolating from Table 4.5,
= erf z
−
= x −
−
2.0
2.2 2.0
x = 2.12 and, t =
ª
¬
346.4
z
º
¼
2
=
ª
¬
346.4
2.12
º
¼
2
= 26,700 s = 7.42 h
4.56 Calculate the diffusivity D in square meters per second for the diffusion of nickel in FCC iron at 1100ºC. Use values of D
0
= 7.7 × 10 –5 /s; Q = 280 kJ/mol;
R = 8.314 J/ (mol· K).
m 2
The diffusivity of the nickel into FCC iron at 1373 K is:
− Q RT = ®
¯
= × − 5 2 -24.53
)
ª
«
¬
[
-280,000 J/mol
]
º
»
¼ ¿
=
4.57 Calculate the diffusivity in m
D
0
= 5.10 × 10 – 4 m 2
2 /s of carbon in HCP titanium at 700ºC. Use
/s; Q = 182 kJ/mol; R = 8.314 J/ (mol· K).
The diffusivity of carbon into HCP titanium is:
− Q RT = − 4 2
ª
«
¬
[
-182,000 J/mol
]
º
»
¼
½
¾
= × − 4 2 -22.49
)
=
4.58 Calculate the diffusivity in m
3.4 × 10 –5 m 2
2 /s for the diffusion of zinc in copper at 350ºC. Use D
0
/s; Q = 191 kJ/mol.
=
The diffusivity of zinc into copper at 623 K is:
Smith Foundations of Materials Science and Engineering 73
− Q RT = − 4 2
ª
«
¬
[
-191,000 J/mol
]
º
»
¼
½
¾
= × − 5 2 -36.88
)
=
4.59 The diffusivity of manganese atoms in the FCC iron lattice is 1.5 × 10 and 1.5 × 10 –15 m 2
–14 m 2 /s at 1300ºC
/s at 400ºC. Calculate the activation energy in kJ/mol for this case in this temperature range. Data: R = 8.314 J/(mol·K).
The activation energy may be calculated using the Arrhenius type equation,
D
D
= = exp
ª
¬
− Q 1
−
1
©
2
T
1 exp( − /
2
) exp( − /
1
) ¹
º
¼ where T
1
= 400 C = 673 K and T
2
= 1300 C = 1573 K. Substituting,
1.5 10
− 14 m /s
1.5 10 − 15 m /s
= exp
ª
¬
− Q
⋅
= × − 4 Q
§
©
1 1 ·
¹
º
¼
= × − 4 Q
Q = 22,518 J/mol = 22.5 kJ/mol
4.60 The diffusivity of copper atoms in the aluminum lattice is 7.5 × 10 –13 m 2 /s at 600ºC and
2.5 × 10 –15 m 2 /s at 400ºC. Calculate the activation energy for this case in this temperature range. [ R = 8.314 J/(mol·K).]
The activation energy associated with copper diffusing into aluminum for this temperature range is,
D
D
= exp
ª
«
¬
− Q §
¨
1
−
1
©
2
T
1
·
¹
º
»
¼ where T
1
= 400 C = 673 K and T
2
= 600 C = 873 K. Substituting,
Smith Foundations of Materials Science and Engineering 74
7.5 10 − 13 m /s
2.5 10 − 15 m /s
= exp
ª
¬
− Q
⋅
= × − 5 Q
§
©
1 1 ·
¹
º
¼
= × − 5 Q
Q = 139,320 J/mol = 139.3 kJ/mol
4.61 The diffusivity of iron atoms in the BCC iron lattice is 4.5 × 10 –23
5.9 × 10 –16 m 2
m 2 /s at 400ºC and
/s at 800ºC. Calculate the activation energy in kJ/mol for this case in this temperature range. [ R = 8.314 J/(mol·K).]
The activation energy associated with iron diffusing into BCC iron for this temperature range is,
D
D
800 C
400 C
= exp
− Q
1
−
1
2
T
1
where T
1
= 400 C = 673 K and T
2
= 800 C = 1073 K. Substituting,
5.9 10 − 16 m /s
4.5 10
− 23 m /s
= exp
− Q
⋅
1 1
= × − 5 Q
= × − 5 Q
Q = 246,007 J/mol = 246 kJ/mol
Smith Foundations of Materials Science and Engineering 75
CHAPTER 8 PHASE DIAGRAMS
8.1 Define (a) a phase in a material and (b) a phase diagram.
(a) A phase in a material is a microscopic region that differs in structure and/or composition from another region.
(b) A phase diagram is a graphical representation of the phases present within a materials system for a range of temperatures, pressures and compositions.
8.2 In the pure water pressure-temperature equilibrium phase diagram (Fig. 8.1) what phases are in equilibrium for the following conditions:
(a) Along the freezing line
(b) Along the vaporization line
(c) At the triple point
(a) Along the freezing line, liquid and solid phases are in equilibrium.
(b) Along the vaporization line, liquid and vapor phases exist in equilibrium.
(c) At the triple point, all three phase – vapor, liquid and solid – coexist.
8.3 How many triple points are there in the pure iron pressure-temperature equilibrium phase diagram of Fig. 8.2? What phases are in equilibrium at each of the triple points?
Three triple points can be identified having the following phases in equilibrium:
1. vapor, liquid and δ Fe
δ Fe, and γ Fe
3. vapor, Fe, α Fe
8.4 Write the equation for Gibbs phase rule and define each of the terms.
The equation for the Gibbs phase rule is:
P + F = C +2 where P = the number of phases that coexist within a specific system
F
C
= the degrees of freedom for the system
= the number of components in the system
8.5 Refer to the pressure-temperature equilibrium phase diagram for pure water (Fig. 8.1) and answer the following:
(a) How many degrees of freedom are there at the triple point?
(b) How many degrees of freedom are there along the freezing line?
Smith Foundations of Materials Science and Engineering Solution Manual 168
(a) At the triple point, there are zero degrees of freedom.
(b) Along the freezing line of pure water, there is one degree of freedom.
8.6 What is a binary isomorphous alloy system?
The binary isomorphous alloy system is a two-component system in which the two elements are completely soluble in each other in the liquid and solid states and form a single type of crystal structure for all compositions.
8.7 What are the four Hum-Rothery rules for the solid solubility of one element in another?
The four Hum-Rothery rules for the solid solubility of one element in another are:
1. The crystal structure of each element of the solid solution must be the same.
2. The size of the atoms of each of the two elements must not differ by more than fifteen percent.
3. The elements should not form compounds with each other; there should be no appreciable difference in the electronegativities of the two elements.
4. The elements should have the same electron valence.
8.8 A number of elements along with their crystal structures and atomic radii are listed in the following table. Which pairs might be expected to have complete solid solubility in each other?
Silver
Crystal
Structure
Atomic radius (nm)
FCC 0.144 Lead
Crystal
Structure
Atomic radius (nm)
FCC 0.175
Copper
Gold
FCC 0.128 Rhodium FCC 0.134
FCC 0.144 Platinum FCC 0.138
Aluminum FCC
Sodium BCC 0.185 Molybdenum BCC 0.136
Pairs of these elements which may be expected to have complete solid solubility in each other are:
Silver–Palladium
Silver–Gold
Copper–Nickel Rhodium–Platinum
8.9 Derive the lever rule for the amount in weight percent of each phase in two-phase regions of a binary phase diagram. Use a phase diagram in which two elements are completely soluble in each other.
Smith Foundations of Materials Science and Engineering Solution Manual 169
The lever-rule equations can be derived by first recognizing that the sum of the weight fractions of the liquid and solid phases which must equal 1.
X l
+ X s
= 1
Considering the weight balance of B in the alloy as a whole and the sum of B in the two phases, we arrive at: w
0
= X w + X w s
Combining these two equations gives w
0 s
) l
+ X w
Solving for the X s
gives the first lever-rule:
Wt fraction of solid phase = X s
= w o
− w l w s
− w l
Similarly, the second lever-rule is found to be:
Wt fraction of liquid phase = X l
= w s
− w
0 w s
− w l
8.10 Consider an alloy containing 70 wt % Ni and 30 wt % Cu (see Fig. 8.3).
(a) At 1350ºC make a phase analysis assuming equilibrium conditions. In the phase analysis include the following:
(i.) What phases are present?
(ii.) What is the chemical composition of each phase?
(iii.) What amount of each phase is present?
(b) Make a similar phase analysis at 1500ºC.
(c) Sketch the microstructure of the alloy at each of these temperatures by using circular microscopic fields.
(a)
(i) The phases present are the liquid and solid ( L + α ).
(ii) The chemical composition of liquid is w l
= 62 wt % Ni while that of the solid is w s
= 74 wt % Ni.
(iii) The weight percent of solid and liquid are:
Wt % of liquid phase = × 100% = 33.3%
Smith
Wt % of solid phase = × 100% = 66.67%
Foundations of Materials Science and Engineering Solution Manual 170
(b) At 1500ºC, the alloy is 100% liquid.
(c) The microstructure of the alloy at these temperatures would look similar to the following sketches.
Solid α phase
100% Liqui d
Liquid phase L
1350ºC 1500ºC
8.11 Describe how the liquidus and solidus of a binary isomorphous phase diagram can be determined experimentally.
The liquidus and solidus of a binary isomorphous phase diagrams can be determined experimentally by measuring cooling rate for several specific alloy compositions and plotting the corresponding liquid-solid curves. The phase diagram can then be constructed by plotting the liquidus and solidus temperatures versus composition of the alloys.
8.12 Explain how a cored structure is produced in a 70% Cu-30% Ni alloy.
A cored structure is produced in a 70% Cu-30% Ni alloy when the alloy is cooled rapidly; without sufficient time for complete solid-state diffusion, concentration gradients remain in the alloy structure.
8.13 How can the cored structure in a 70% Cu-30% Ni alloy be eliminated by heat treatment?
The cored structure can be eliminated in ingots and castings by heat treating at elevated temperatures. This homogenization process accelerates the required solid-state diffusion and thus produces a homogeneous structure in the alloy.
8.14 Explain what is meant by the term liquation. How can a liquated structure be produced in an alloy? How can it be avoided?
Liquation is the localized melting which occurs if an alloy is heated to a temperature greater than the lowest melting temperature of the alloy’s constituents. The result of such overheating is a liquated structure, in which grain boundaries may be melted. To avoid liquation, the heat treatment should be performed such that the melting temperature is approached slowly but never exceeded.
Smith Foundations of Materials Science and Engineering Solution Manual 171
Figure 8.31 The copper-silver phase diagram.
8.15 Consider the binary eutectic copper-silver phase diagram in Fig. 8.31. Make phase analyses of an 88 wt % Ag-12 wt % Cu alloy at the temperatures (a) 1000ºC, (b) 800ºC,
(c) 780 C + T − ∆
(i) The phases present
In the phase analysis, include:
(ii) The chemical compositions of the phases
(iii) The amounts of each phase
(iv) Sketch the microstructure by using 2 cm diameter circular fields.
(a) At 1000ºC:
Phases present: liquid
Compositions of phases: 100%
(b) At 800ºC,
Compositions of phases: 78% Ag in liquid phase 93% Ag in β phase
Smith Foundations of Materials Science and Engineering Solution Manual 172
Amounts of phases:
Wt % liquid phase =
Wt % beta phase =
× 100% = 33.3%
× 100% = 66.6%
,
Phases present: liquid
Compositions of phases: 71.9% Ag in liquid phase
Amounts of phases: beta
91.2% Ag in β phase
Wt % liquid phase = × 100% = 16.6%
× 100% = 83.4% Wt % beta phase =
(d) At 780 C T,
Compositions of phases: 7.9% Ag in α phase
Amounts of phases:
Wt % alpha phase =
Wt % beta phase =
× 100% = 3.84%
× 100% = 96.16%
91.2% Ag in β phase
8.16 If 500g of a 40 wt % Ag-60 wt % Cu alloy is slowly cooled from 1000ºC to just below
780ºC (see Fig. 8.31):
(a) How many grams of liquid and proeutectic alpha are present at 850ºC?
?
(a) At 850ºC,
Wt % liquid = × =
Smith Foundations of Materials Science and Engineering Solution Manual 173
Wt % proeutectic α = ×
Weight of proeutectic α =
×
×
(b) In the eutectic structure at 780 C + T ,
Wt % liquid = × =
=
=
364 g
136 g
Wt % proeutectic α = ×
251 g
249 g Weight of proeutectic α =
×
×
=
=
(c) In the eutectic structure at 780 C T, the number of grams of α present is,
Wt % total α = ×
Weight of total α = × = 307.5 g
(d) In the eutectic structure at 780 C T, the number of grams of β present is,
Wt % total β = ×
Weight of β = × = 192.5 g
8.17 A lead-tin (Pb-Sn) alloy consists of 60 wt % proeutectic β and 60 wt % eutectic α β at
− ∆ T .
Calculate the average composition of this alloy (see Fig. 8.11).
Since the alloy contains 60 wt % proeutectic β , the wt % Sn must lie between 61.9 wt % and 97.5 wt %:
% proeutectic β = x − 61.9
= 0.60
x =
Thus, the alloy consists of 83.3 % Sn and 16.7 % Pb .
8.18 A Pb-Sn alloy (Fig. 8.11) contains 40 wt % β and 60 wt % α at 50ºC. What is the average composition of Pb and Sn in this alloy?
Smith Foundations of Materials Science and Engineering Solution Manual 174
At 50ºC, the phase compositions are 100% Sn for β and approximately 2% Sn for α .
Thus,
% α =
100.0
− x
= 0.60, x = =
The alloy consists of 41.2 % Sn and 58.8 % Pb .
8.19 An alloy of 30 wt % Pb and 70 wt % Sn is slowly cooled from 250ºC to 27ºC (see Fig.
8.11).
(a) Is this alloy hypoeutectic or hypereutectic?
(b) What is the composition of the first solid to form?
(c) What are the amounts and composition of each phase that is present at
(d) What is the amount and composition of each phase that is present at
(e) What are the amounts of each phase present at room temperature?
183 C
183 C
+ ∆ T
− ∆ T ?
?
(a) This alloy is hypereutectoid; the composition lies to the right of the eutectic point.
(b) The first solid to form is solid solution β containing approximately 98 % Sn.
(c) At + ∆ T , the compositions of the phases present are 61.9% Sn in liquid phase and 19.2% Sn in beta phase. The amounts of the respective phases present are:
Wt % liquid =
Wt % beta =
× 100% = 77.2%
× 100% = 22.8%
(d) At 183 C − ∆ T , the compositions of the phases present are 19.2 % Sn in
97.5 % Sn in β phase. The amounts of the respective phases present are:
α phase and
Wt % total α = × 100% = 35.1%
Wt % total beta = × 100% = 64.8%
(e) As the alloy is cooled below the eutectic temperature, the tin content in the alpha phase and the lead content in the beta phase are further reduced. However, at room temperature (20ºC), equilibrium is not achieved because the diffusion rate is so slow.
Referring to Fig. 8.11, if the solvus line is extrapolated to 20ºC, the approximate composition of alpha and beta are 2.0% and 100.0 %, respectively. Thus,
Smith Foundations of Materials Science and Engineering Solution Manual 175
Wt % total α =
Wt % total beta =
× 100% = 30.6%
× 100% = 69.4%
Figure 8.32 The iridium-osmium phase diagram.
8.20 Consider the binary peritectic iridium-osmium phase diagram of Fig. 8.32. Make phase analyses of a 70 wt % Ir-30 wt % Os at the temperatures (a) 2600ºC (b) 2665 C , and (c) 2665 C − ∆ T .
In the phase analyses include:
(i.) The phases present
(ii.) The chemical compositions of the phases
(iii.) The amounts of each phase
(iv.) Sketch the microstructure by using 2 cm diameter circular fields.
(a) At 2600ºC,
(i) Phases Present: Liquid and alpha phases
(ii) Compositions of Phases: 16% Os in liquid phase; 38% Os in alpha phase
(iii) Amounts of phases:
Wt % alpha = × 100% = 63.6%
Solid α phase
Wt % liquid = × 100% = 36.4%
Liquid phase L
Smith Foundations of Materials Science and Engineering Solution Manual 176
(b) At 2665 C ,
(i) Phases Present: Liquid and beta phases
(ii) Compositions of Phases: 23% Os in liquid phase; 61.5% Os in β phase
(iii) Amounts of phases:
Wt % beta = × 100% = 18.2%
Solid β phase
Wt % liquid = × 100% = 81.8%
Liquid phase L
(c) At 2665 C ,
(i) Phases Present: Liquid and alpha phases
(ii) Compositions of Phases: 23% Os in liquid phase; 43% Os in α phase
(iii) Amounts of phases:
Wt % alpha = × 100% = 35.0%
Solid α phase
Wt % liquid = × 100% = 65.0%
Liquid phase L
8.21 Consider the binary peritectic iridium-osmium phase diagram of Fig. 8.32. Make phase analyses of a 40 wt % Ir-60 wt % Os at the temperatures (a) 2600ºC (b) 2665 C , and (c) 2665 C ,
Prob. 8.20.
(d) 2800ºC. Include in the phase analyses the four items listed in
(a) At 2600ºC,
(i) Phases Present: Alpha and beta phases
(ii) Compositions of Phases: 43% Os in alpha phase; 61.5% Os in beta phase
(iii) Amounts of phases:
Wt % alpha = × 100% = 8.1%
Solid α phase
Wt % beta = × 100% = 91.9%
Beta phase
(b) At 2665 C ,
(i) Phases Present: Liquid and beta phases
(ii) Compositions of Phases: 23% Os in alpha phase; 61.5% Os in beta phase
(iii) Amounts of phases:
Wt % liquid phase = × 100% = 3.9%
Solid β phase
Wt % beta phase = × 100% = 96.1%
Liquid phase
Smith Foundations of Materials Science and Engineering Solution Manual 177
(c) At 2665 C ,
(i) Phases Present: Alpha and beta phases
(ii) Compositions of Phases: 43% Os in liquid phase; 61.5% Os in beta phase
(iii) Amounts of phases:
Wt % alpha = × 100% = 8.1%
Solid α phase
Wt % beta = × 100% = 91.9%
Beta phase
(d) At 2800ºC,
(i) Phases Present: Alpha and beta phases
(ii) Compositions of Phases: 45% Os in liquid phase; 85% Os in beta phase
(iii) Amounts of phases:
Wt % liquid = × 100% = 62.5%
Solid α phase
Wt % beta = × 100% = 37.5%
Beta phase
8.22 Describe the mechanism that produces the phenomenon of surrounding in a peritectic alloy which is rapidly solidified through the peritectic reaction.
Surrounding in a rapidly solidified peritectic alloy is a nonequilibrium phenomenon in which the alpha phase is encased by the beta phase during the peritectic reaction. As a result, the solid beta phase acts as a barrier to alpha diffusion and the peritectic reaction rate decreases continuously.
8.23 Can coring and surrounding occur in a peritectic-type alloy which is rapidly solidified?
Explain.
In a rapidly solidified peritectic alloy, coring can occur during the formation of the primary alpha phase and subsequently, the cored alpha phase can be surrounded by the beta phase during the peritectic reaction.
8.24 Consider an Fe-4.2 wt % Ni alloy (Fig. 8.16) that is slowly cooled from 1550 to 1450ºC.
What weight percent of the alloy solidifies by the peritectic reaction?
Referring to Fig. 8.16 on the following page, during the peritectic reaction, liquid and δ phases react to form solid γ :
Wt % γ = × 100% = 66.7%
At the end of the reaction, there is an excess of δ phase having a wt % of 33.3 %.
Smith Foundations of Materials Science and Engineering Solution Manual 178
Figure 8.16 The peritectic region of the iron-nickel phase diagram.
The peritectic point is located at 4.3% Ni and 1517°C, which is point c.
8.25 Consider an Fe-5.0 wt % Ni alloy (Fig. 8.16) that is slowly cooled from 1550 to 1450ºC.
What weight percent of the alloy solidifies by the peritectic reaction?
During the peritectic reaction, liquid solidifies to form solid γ :
Wt % γ = × 100% = 36.4%
8.26 Determine the weight percent and composition in weight percent of each phase present in
+ ∆ T .
Just above the eutectic temperature, the alloy composition is: 4.0 wt % Ni in the δ phase and 5.4 wt % Ni in the liquid phase. The weight percentages of these phases are:
Wt % liquid =
Wt % δ =
× 100% = 14.3%
× 100% = 85.7%
Smith Foundations of Materials Science and Engineering Solution Manual 179
8.27 Determine the composition in weight percent of the alloy in the Fe-Ni system (Fig. 8.16) that will produce a structure of 40 wt % δ and 60 wt % γ , just below the peritectic temperature.
For a 40 wt % δ composition to exist at 1517 C
Wt % δ =
4.3
− x
= 0.40
x =
− ∆ T ,
4.18%
Thus, the alloy contains 4.18% Ni and 95.82% Fe .
8.28 What is a monotectic invariant reaction? How is the monotectic reaction in the copperlead system important industrially?
A monotectic invariant reaction is one in which a liquid phase reacts isothermally to form a solid phase and a new liquid phase. The monotectic reaction is important industrially to the copper-lead system because it can produce a nearly pure lead phase in copper-zinc brasses which improves the machining properties of the alloys; the lead sufficiently reduces the ductility of the alloys to cause machined chips to naturally break away from the workpiece.
8.29 In the copper-lead (Cu-Pb) system (Fig. 8.23) for an alloy of Cu-10 wt % Pb, determine the amounts and compositions of the phases present at (a) 1000ºC (b) 955 C , (c)
and (d) 200ºC.
(a) At 1000ºC,
Compositions of Phases:
Amounts of Phases:
100% Cu, 0% Pb in
81% Cu, 19% Pb in
α
L
phase;
1
phase
Wt % α = × 100% = 47.4% Wt % L
1
= × 100% = 52.6%
(b) At 955 C ,
Compositions of Phases:
Amounts of Phases:
100% Cu, 0% Pb in
64% Cu, 36% Pb in
α
L
1 phase;
phase
Wt % α = × 100% =
(c) At 955 C ,
Compositions of Phases:
72.2% Wt % L
1
=
100% Cu, 0% Pb in
13% Cu, 87% Pb in
α
L
phase;
2
phase
× 100% = 27.8%
Smith Foundations of Materials Science and Engineering Solution Manual 180
Amounts of Phases:
Wt % α = × 100% = 88.5% Wt % L
2
= × 100% = 11.5%
(d) At 200ºC,
Compositions of Phases:
Amounts of Phases:
Wt % α =
99.995% Cu, 0.005% Pb in
0.007% Cu, 99.993% Pb in
× 100% = 90% Wt % β =
α
β phase;
phase
× 100% = 10%
Smith
Figure 8.23 The copper-lead phase diagram.
Foundations of Materials Science and Engineering Solution Manual 181
8.30 For an alloy Cu-70 wt % Pb (Fig. 8.23), determine the amounts and compositions in weight percent of the phases present at (a) 955 C , (b) 955 C , and (c) 200ºC.
(a) At 955 C ,
Compositions of Phases:
Amounts of Phases:
64% Cu, 36% Pb in L
13% Cu, 87% Pb in L
2
1
phase
phase;
Wt % L
2
= × 100% =
(b) At 955 C ,
Compositions of Phases:
Amounts of Phases:
66.7% Wt % L
1
=
100% Cu, 0% Pb in α phase;
13% Cu, 87% Pb in L
2
phase
× 100% = 33.3%
Wt % α = × 100% = 19.5% Wt % L
2
= × 100% = 80.5%
(c) At 200ºC,
Compositions of Phases:
Amounts of Phases:
Wt % α = × 100% =
99.995% Cu, 0.005% Pb in
0.007% Cu, 99.993% Pb in
30% Wt % β =
α
β
phase;
phase
× 100% = 70%
8.31 What is the average composition (weight percent) of a Cu-Pb alloy that contains 30 wt %
L
1
and 70 wt % α at 955 C + ∆ T ?
For a 30 wt % L
1 composition to exist at 955 C
Wt % L
1
= x − 0
= 0.30
+ ∆ T , x = 10.8%
Thus, the alloy contains 10.8% Pb and 89.2% Cu .
Smith Foundations of Materials Science and Engineering Solution Manual 182
8.32 Write equations for the following invariant reactions: eutectic, eutectoid, peritectic, and peritectoid. How many degrees of freedom exist at invariant reaction points in binary phase diagrams?
Eutectic Reaction: L α + β
α → β γ Eutectoid Reaction:
Peritectic Reaction: α + L → β
Peritectoid Reaction: → γ
There are zero degrees of freedom at the invariant reaction points in binary phase diagrams.
8.33 How are eutectic and eutectoid reactions similar? What is the significance of the – oid suffix?
The eutectic and eutectoid reactions are similar in that they both involve the decomposition of a single phase into two solid phases. The – oid suffix indicates that a solid, rather than liquid, phase is decomposing.
8.34 Distinguish between (a) a terminal phase and (b) an intermediate phase.
A terminal solid solution phase occurs at the end of a phase diagram, bordering on pure components. Whereas, an intermediate solid solution phase occurs within a composition range inside the phase diagram and is separated from other phases in a binary diagram by two-phase regions.
8.35 Distinguish between (a) an intermediate phase and (b) an intermediate compound.
Intermediate phases, which may occur in binary metal or ceramic phase diagrams, represent a range of solid solution compositions. Conversely, an intermediate compound has a fixed composition and definite stoichiometry at room temperature and is formed between two metals or a metal and a nonmetal.
8.36 What is the difference between a congruently melting compound and an incongruently melting one?
A congruently melting compound maintains its composition right up to its melting point.
Whereas an incongruently melting compound undergoes peritectic decomposition upon heating; the solid compound decomposes into a liquid and another solid solution.
8.37 Consider the Cu-Zn phase diagram of Fig. 8.25.
(a) What is the maximum solid solubility in weight percent of Zn in Cu in the terminal solid solution α ?
(b) Identify the intermediate phases in the Cu-Zn phase diagram.
Smith Foundations of Materials Science and Engineering Solution Manual 183
(c) Identify the three-phase invariant reactions in the Cu-Zn diagram.
(i) Determine the composition and temperature coordinates of the invariant reactions.
(ii) Write the equations for the invariant reactions.
(iii) Name the invariant reactions.
(a) The maximum solid solubility in weight percent of zinc in copper in the solid solution
α is 39%.
(b) The intermediate phases are β , γ , δ , and ∫ .
(c) The three-phase invariant reactions are:
1. Peritectic reaction at 903ºC, 36.8% Zn
α
2. Peritectic reaction at 835ºC, 59.8% Zn
β (56.5% Zn) + L (59.8% Zn)
3. Peritectic reaction at 700ºC, 73% Zn
γ
β (36.8% Zn)
(59.8% Zn)
γ (69.8% Zn) + L (80.5% Zn)
4. Peritectic reaction at 598ºC, 78.6% Zn
δ (73% Zn)
δ (76.5% Zn) + L (89% Zn) ò (78.6% Zn)
5. Peritectic reaction at 424ºC, 97.3% Zn
ò (87.5% Zn) + L (98.3% Zn)
6. Eutectoid reaction at 558ºC, 73% Zn
η (97.3% Zn)
δ (73% Zn) γ (69.8% Zn) + ò (78.6% Zn)
7. Eutectoid reaction at 250ºC, 47% Zn
β ′ (47% Zn) α (37% Zn) + γ (59% Zn)
8.38 Consider the aluminum-nickel (Al-Ni) phase diagram of Fig. 8.33 on the following page.
For this phase diagram:
(a) Determine the coordinates of the composition and temperature of the invariant reactions.
(b) Write the equations for the three-phase invariant reactions and name them.
(c) Label the two-phase regions in the phase diagram.
(a) and (b):
1. Eutectic reaction at 639ºC, 0.1% Ni
L (0.1% Ni)
Smith Foundations of Materials Science and Engineering Solution Manual 184
2. Peritectic reaction at 854ºC, 42% Ni
L
3. Peritectic reaction at 1133ºC, 59% Ni
L
4. Peritectoid reaction at 700ºC, 81% Ni
5. Peritectic reaction at 1395ºC, 86% Ni
L
6. Eutectic reaction at 1385ºC, 86% Ni
L (87% Ni)
(c) The two-phase regions are identified in Fig. 8.33 below.
Smith Foundations of Materials Science and Engineering Solution Manual 185
Figure 8.34 Nickel-vanadium phase diagram.
8.39 Consider the nickel-vanadium (Ni-V) phase diagram of Fig. 8.34. For this phase diagram repeat questions (a), (b), and (c) of Prob. 8.38.
(c) The two-phase regions are identified in Fig. 8.34 above.
(a) and (b):
1. Eutectoid reaction at 906ºC, 29.5% V
Ni (29.5% V)
2
2. Eutectoid reaction at 890ºC, 35.2% V
Ni (35.2% V) + σ ′ (51% V)
3. Eutectic reaction at 1202ºC, 47.5% V
L (47% V) Ni (40% V) + σ ′
4. Peritectic reaction at 1280ºC, 64% V
(51% V)
Smith Foundations of Materials Science and Engineering Solution Manual 186
L
5. Peritectoid reaction at 900ºC, 75.3% V
σ ′ (64% V)
σ ′
Figure 8.35 Titanium-aluminum phase diagram.
8.40 Consider the titanium-aluminum (Ti-Al) phase diagram of Fig. 8.35. For this phase diagram, repeat questions (a), (b), and (c) of Prob. 8.38.
(c) The two-phase regions are identified in Fig. 8.35 above.
(a) and (b):
1. Peritectoid reaction at 1285ºC, 32% Al
β
2. Eutectoid reaction at 1125ºC, 27% Al
α Ti (27% Al)
α Ti (32% Al)
Smith Foundations of Materials Science and Engineering Solution Manual 187
3. Peritectic reaction at 1380ºC, 58% Al
L
4. Peritectic reaction at 1350ºC, 63% Al
δ
L (69% Al) + δ (60% Al)
5. Peritectoid reaction at 1240ºC, 55% Al
TiAl (51% Al) + δ (57% Al)
6. Eutectoid reaction at 1150ºC, 58% Al
δ (58% Al)
7. Peritectic reaction at 665ºC, 99% Al
(58% Al)
3
L Al (99% Al)
8.41 What is the composition of point y in Fig. 8.29?
As indicated in the ternary diagram figure below, the composition of point y is 20% A,
30% B and 50% C.
30%
Smith
50%
Foundations of Materials Science and Engineering Solution Manual
20%
188