PT Groups and Trends
advertisement

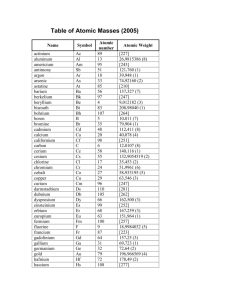
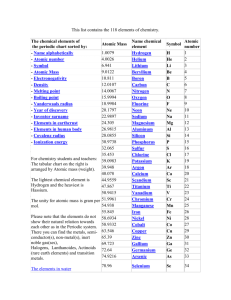
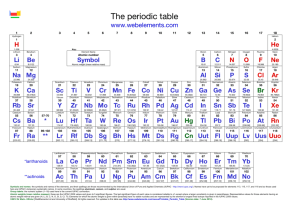
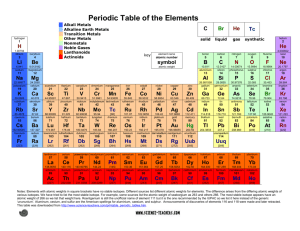
1/27 - 1/28 Bell ringer... • Identify the following: ✦ What do the electron configurations of elements in the same row (horizontal) have in common? ✦ ✦ Elements in the same column (vertical)? What difference does this make? Groups, periods, and trends! Organizing the periodic table • Mendeleev’s big break in the organization of the elements came from recognizing patterns in the periodic table • Early elements repeat their properties and bonding every eight elements • Organizing the periodic table with respect to these repetitions created at a table with stacks of rows Periodicity... • the quality or character of being periodic; the tendency to recur at intervals • The periodic table is arranged in rows of repeating electron sequences due to the periodicity of subshells within the electron cloud e l p o e ! p d e e s s u u a f c n e o B c d e m e se Naming (just because?) • Periods • In chemistry the rows of the periodic table are referred to as periods • Groups • Columns on the periodic table are referred to as groups Periods • Elements in the same period on the periodic table share the same number of energy levels (shells) • Periods with d and f subshells display great similarity • The first few periods have few common properties Naming (just because?) • Groups • Groups have the same number of electrons in their • Columns on the periodic table are referred to as groups outermost electron sublevel • Due to this similarity elements within a group will e b share similar reactivity and thus properties o s l a n n o a c k c p o u l o b r N ot entirely offici l g a t al A i ! ! b ! r T o P --> e an h t Alkali Metals (1A) ✦ One electron in outer (s) orbital ✦ Very Reactive ✦ Malleable, ductile, conductive ✦ Can burn or explode when exposed to water Alkali Metals (1A) Prof Mokeur's Periodic Table IA H1 VIIIA He 2 1 Hydrogen IIA Li 3 Be 4 H1 B5 Lithium Beryllium Hydrogen Boron IIIA IVA C6 VA N7 VIA VIIA F9 Ne 10 Carbon Nitrogen Oxygen Fluorine Neon P 15 S 16 Cl 17 Ar 18 Phosphorus Sulfur Chlorine O8 Helium 2 Na 11 Mg 12 Al 13 Si 14 3 Sodium K 19 Magnesium IIIB IVB VB Ca 20 Sc 21 Ti 22 V 23 VIB VIIB VIII IB IIB Aluminum Silicon Cr 24 Mn 25 Fe 26 Co 27 Ni 28 Cu 29 Zn 30 Ga 31 Ge 32 As 33 Argon Se 34 Br 35 Kr 36 4 Potassium Calcium Rb 37 Sr 38 Scandium Y 39 Titanium Vanadium Chromium Manganese Iron Strontium Yttrium Nickel Copper Zinc Gallium Germanium Arsenic Selenium Zr 40 Nb 41 Mo 42 Tc 43 Ru 44 Rh 45 Pd 46 Ag 47 Cd 48 In 49 Sn 50 Sb 51 Te 52 5 Rubidium Cobalt Zirconium Niobium 4,3 Molybdenum Technetium Ruthenium Rhodium Palladium Silver Cadmium 4,2 -4 Tin Indium 5,3 -3 Antimony Tellurium Bromine Krypton I 53 Xe 54 Xenon Iodine Cs 55 Ba 56 La 57 Hf 72 Ta 73 W 74 Re 75 Os 76 Ir 77 Pt 78 Au 79 Hg 80 Tl 81 Pb 82 Bi 83 Po 84 At 85 Rn 86 Cesium Platinum 6 Barium Lanthanum Hafnium Tantalum Tungsten Rhenium Osmium Iridium Gold Mercury Thallium Lead Bismuth Polonium Fr 87 Ra 88 Ac 89 Rf 104 Db 105 Sg 106 Bh 107 Hs 108 Mt 109 Ds 110 Rg 111 Cn 112 Uut 113 114 Uup 115 Astatine Radon 116 Uus 117 Uuo 118 7 Francium Radium Actinium Rutherfordium Dubnium Seaborgium Bohrium Hassium Meitnerium Darmstadtium Roentgenium Copernicium Ununtrium Ununpentium Ununseptium Ununoctium Ce 58 Pr 59 Nd 60 Pm 61 Sm 62 Eu 63 Gd 64 Tb 65 Dy 66 Ho 67 Er 68 Tm 69 Yb 70 Lu 71 6 Cerium Praseodymium Neodymium Promethium Samarium Europium Gadolinium Terbium Dysprosium Holmium Erbium Thulium Ytterbium Lutetium Th 90 Pa 91 U 92 Np 93 Pu 94 Am 95 Cm 96 Bk 97 Cf 98 Es 99 Fm 100 Md 101 No 102 Lr 103 7 Thorium Protactinium Uranium Neptunium Plutonium Americium Curium Berkelium Californium Einsteinium Fermium Mendelevium Nobelium Lawrencium Alkaline Earth Metals (IIA) ✦ Two electrons in outer (s) orbitals ✦ Smaller atomic radii than alkali metals ✦ Malleable, ductile, conductive ✦ Readily form ions (cations) Alkaline Earth Metals (IIA) Prof Mokeur's Periodic Table IA H1 VIIIA He 2 1 Hydrogen IIA Li 3 Be 4 H1 B5 Lithium Beryllium Hydrogen Boron IIIA IVA C6 VA N7 VIA VIIA F9 Ne 10 Carbon Nitrogen Oxygen Fluorine Neon P 15 S 16 Cl 17 Ar 18 Phosphorus Sulfur Chlorine O8 Helium 2 Na 11 Mg 12 Al 13 Si 14 3 Sodium K 19 Magnesium IIIB IVB VB Ca 20 Sc 21 Ti 22 V 23 VIB VIIB VIII IB IIB Aluminum Silicon Cr 24 Mn 25 Fe 26 Co 27 Ni 28 Cu 29 Zn 30 Ga 31 Ge 32 As 33 Argon Se 34 Br 35 Kr 36 4 Potassium Calcium Rb 37 Sr 38 Scandium Y 39 Titanium Vanadium Chromium Manganese Iron Strontium Yttrium Nickel Copper Zinc Gallium Germanium Arsenic Selenium Zr 40 Nb 41 Mo 42 Tc 43 Ru 44 Rh 45 Pd 46 Ag 47 Cd 48 In 49 Sn 50 Sb 51 Te 52 5 Rubidium Cobalt Zirconium Niobium 4,3 Molybdenum Technetium Ruthenium Rhodium Palladium Silver Cadmium 4,2 -4 Tin Indium 5,3 -3 Antimony Tellurium Bromine Krypton I 53 Xe 54 Xenon Iodine Cs 55 Ba 56 La 57 Hf 72 Ta 73 W 74 Re 75 Os 76 Ir 77 Pt 78 Au 79 Hg 80 Tl 81 Pb 82 Bi 83 Po 84 At 85 Rn 86 Cesium Platinum 6 Barium Lanthanum Hafnium Tantalum Tungsten Rhenium Osmium Iridium Gold Mercury Thallium Lead Bismuth Polonium Fr 87 Ra 88 Ac 89 Rf 104 Db 105 Sg 106 Bh 107 Hs 108 Mt 109 Ds 110 Rg 111 Cn 112 Uut 113 114 Uup 115 Astatine Radon 116 Uus 117 Uuo 118 7 Francium Radium Actinium Rutherfordium Dubnium Seaborgium Bohrium Hassium Meitnerium Darmstadtium Roentgenium Copernicium Ununtrium Ununpentium Ununseptium Ununoctium Ce 58 Pr 59 Nd 60 Pm 61 Sm 62 Eu 63 Gd 64 Tb 65 Dy 66 Ho 67 Er 68 Tm 69 Yb 70 Lu 71 6 Cerium Praseodymium Neodymium Promethium Samarium Europium Gadolinium Terbium Dysprosium Holmium Erbium Thulium Ytterbium Lutetium Th 90 Pa 91 U 92 Np 93 Pu 94 Am 95 Cm 96 Bk 97 Cf 98 Es 99 Fm 100 Md 101 No 102 Lr 103 7 Thorium Protactinium Uranium Neptunium Plutonium Americium Curium Berkelium Californium Einsteinium Fermium Mendelevium Nobelium Lawrencium Transition Metals ✦ 1-10 electrons in outer (d) orbitals ✦ Fairly unreactive; due to outer orbital being d ✦ Hard, shiny, malleable, ductile, conductive ✦ ***variable oxidation states (more on this later)*** Transition Metals Prof Mokeur's Periodic Table IA H1 VIIIA He 2 1 Hydrogen IIA Li 3 Be 4 H1 B5 Lithium Beryllium Hydrogen Boron IIIA IVA C6 VA N7 VIA VIIA F9 Ne 10 Carbon Nitrogen Oxygen Fluorine Neon P 15 S 16 Cl 17 Ar 18 Phosphorus Sulfur Chlorine O8 Helium 2 Na 11 Mg 12 Al 13 Si 14 3 Sodium K 19 Magnesium IIIB IVB VB Ca 20 Sc 21 Ti 22 V 23 VIB VIIB VIII IB IIB Aluminum Silicon Cr 24 Mn 25 Fe 26 Co 27 Ni 28 Cu 29 Zn 30 Ga 31 Ge 32 As 33 Argon Se 34 Br 35 Kr 36 4 Potassium Calcium Rb 37 Sr 38 Scandium Y 39 Titanium Vanadium Chromium Manganese Iron Strontium Yttrium Nickel Copper Zinc Gallium Germanium Arsenic Selenium Zr 40 Nb 41 Mo 42 Tc 43 Ru 44 Rh 45 Pd 46 Ag 47 Cd 48 In 49 Sn 50 Sb 51 Te 52 5 Rubidium Cobalt Zirconium Niobium 4,3 Molybdenum Technetium Ruthenium Rhodium Palladium Silver Cadmium 4,2 -4 Tin Indium 5,3 -3 Antimony Tellurium Bromine Krypton I 53 Xe 54 Xenon Iodine Cs 55 Ba 56 La 57 Hf 72 Ta 73 W 74 Re 75 Os 76 Ir 77 Pt 78 Au 79 Hg 80 Tl 81 Pb 82 Bi 83 Po 84 At 85 Rn 86 Cesium Platinum 6 Barium Lanthanum Hafnium Tantalum Tungsten Rhenium Osmium Iridium Gold Mercury Thallium Lead Bismuth Polonium Fr 87 Ra 88 Ac 89 Rf 104 Db 105 Sg 106 Bh 107 Hs 108 Mt 109 Ds 110 Rg 111 Cn 112 Uut 113 114 Uup 115 Astatine Radon 116 Uus 117 Uuo 118 7 Francium Radium Actinium Rutherfordium Dubnium Seaborgium Bohrium Hassium Meitnerium Darmstadtium Roentgenium Copernicium Ununtrium Ununpentium Ununseptium Ununoctium Ce 58 Pr 59 Nd 60 Pm 61 Sm 62 Eu 63 Gd 64 Tb 65 Dy 66 Ho 67 Er 68 Tm 69 Yb 70 Lu 71 6 Cerium Praseodymium Neodymium Promethium Samarium Europium Gadolinium Terbium Dysprosium Holmium Erbium Thulium Ytterbium Lutetium Th 90 Pa 91 U 92 Np 93 Pu 94 Am 95 Cm 96 Bk 97 Cf 98 Es 99 Fm 100 Md 101 No 102 Lr 103 7 Thorium Protactinium Uranium Neptunium Plutonium Americium Curium Berkelium Californium Einsteinium Fermium Mendelevium Nobelium Lawrencium Other (basic) metals N ot a literal “gro u p” • Outer orbitals in the p-block • Solids, reactive, generally high density • Not a boring group just a somewhat diverse one... Other (basic) metals Prof Mokeur's Periodic Table IA H1 VIIIA He 2 1 Hydrogen IIA Li 3 Be 4 H1 B5 Lithium Beryllium Hydrogen Boron IIIA IVA C6 VA N7 VIA VIIA F9 Ne 10 Carbon Nitrogen Oxygen Fluorine Neon P 15 S 16 Cl 17 Ar 18 Phosphorus Sulfur Chlorine O8 Helium 2 Na 11 Mg 12 Al 13 Si 14 3 Sodium K 19 Magnesium IIIB IVB VB Ca 20 Sc 21 Ti 22 V 23 VIB VIIB VIII IB IIB Aluminum Silicon Cr 24 Mn 25 Fe 26 Co 27 Ni 28 Cu 29 Zn 30 Ga 31 Ge 32 As 33 Argon Se 34 Br 35 Kr 36 4 Potassium Calcium Rb 37 Sr 38 Scandium Y 39 Titanium Vanadium Chromium Manganese Iron Strontium Yttrium Nickel Copper Zinc Gallium Germanium Arsenic Selenium Zr 40 Nb 41 Mo 42 Tc 43 Ru 44 Rh 45 Pd 46 Ag 47 Cd 48 In 49 Sn 50 Sb 51 Te 52 5 Rubidium Cobalt Zirconium Niobium 4,3 Molybdenum Technetium Ruthenium Rhodium Palladium Silver Cadmium 4,2 -4 Tin Indium 5,3 -3 Antimony Tellurium Bromine Krypton I 53 Xe 54 Xenon Iodine Cs 55 Ba 56 La 57 Hf 72 Ta 73 W 74 Re 75 Os 76 Ir 77 Pt 78 Au 79 Hg 80 Tl 81 Pb 82 Bi 83 Po 84 At 85 Rn 86 Cesium Platinum 6 Barium Lanthanum Hafnium Tantalum Tungsten Rhenium Osmium Iridium Gold Mercury Thallium Lead Bismuth Polonium Fr 87 Ra 88 Ac 89 Rf 104 Db 105 Sg 106 Bh 107 Hs 108 Mt 109 Ds 110 Rg 111 Cn 112 Uut 113 114 Uup 115 Astatine Radon 116 Uus 117 Uuo 118 7 Francium Radium Actinium Rutherfordium Dubnium Seaborgium Bohrium Hassium Meitnerium Darmstadtium Roentgenium Copernicium Ununtrium Ununpentium Ununseptium Ununoctium Ce 58 Pr 59 Nd 60 Pm 61 Sm 62 Eu 63 Gd 64 Tb 65 Dy 66 Ho 67 Er 68 Tm 69 Yb 70 Lu 71 6 Cerium Praseodymium Neodymium Promethium Samarium Europium Gadolinium Terbium Dysprosium Holmium Erbium Thulium Ytterbium Lutetium Th 90 Pa 91 U 92 Np 93 Pu 94 Am 95 Cm 96 Bk 97 Cf 98 Es 99 Fm 100 Md 101 No 102 Lr 103 7 Thorium Protactinium Uranium Neptunium Plutonium Americium Curium Berkelium Californium Einsteinium Fermium Mendelevium Nobelium Lawrencium Metalloids N ot a literal “gro u p” • Some are semi-conductors (conduct heat and electricity • Line separating metals from non-metals interesting ways) • Very useful group Metalloids Prof Mokeur's Periodic Table IA H1 VIIIA He 2 1 Hydrogen IIA Li 3 Be 4 H1 B5 Lithium Beryllium Hydrogen Boron IIIA IVA C6 VA N7 VIA VIIA F9 Ne 10 Carbon Nitrogen Oxygen Fluorine Neon P 15 S 16 Cl 17 Ar 18 Phosphorus Sulfur Chlorine O8 Helium 2 Na 11 Mg 12 Al 13 Si 14 3 Sodium K 19 Magnesium IIIB IVB VB Ca 20 Sc 21 Ti 22 V 23 VIB VIIB VIII IB IIB Aluminum Silicon Cr 24 Mn 25 Fe 26 Co 27 Ni 28 Cu 29 Zn 30 Ga 31 Ge 32 As 33 Argon Se 34 Br 35 Kr 36 4 Potassium Calcium Rb 37 Sr 38 Scandium Y 39 Titanium Vanadium Chromium Manganese Iron Strontium Yttrium Nickel Copper Zinc Gallium Germanium Arsenic Selenium Zr 40 Nb 41 Mo 42 Tc 43 Ru 44 Rh 45 Pd 46 Ag 47 Cd 48 In 49 Sn 50 Sb 51 Te 52 5 Rubidium Cobalt Zirconium Niobium 4,3 Molybdenum Technetium Ruthenium Rhodium Palladium Silver Cadmium 4,2 -4 Tin Indium 5,3 -3 Antimony Tellurium Bromine Krypton I 53 Xe 54 Xenon Iodine Cs 55 Ba 56 La 57 Hf 72 Ta 73 W 74 Re 75 Os 76 Ir 77 Pt 78 Au 79 Hg 80 Tl 81 Pb 82 Bi 83 Po 84 At 85 Rn 86 Cesium Platinum 6 Barium Lanthanum Hafnium Tantalum Tungsten Rhenium Osmium Iridium Gold Mercury Thallium Lead Bismuth Polonium Fr 87 Ra 88 Ac 89 Rf 104 Db 105 Sg 106 Bh 107 Hs 108 Mt 109 Ds 110 Rg 111 Cn 112 Uut 113 114 Uup 115 Astatine Radon 116 Uus 117 Uuo 118 7 Francium Radium Actinium Rutherfordium Dubnium Seaborgium Bohrium Hassium Meitnerium Darmstadtium Roentgenium Copernicium Ununtrium Ununpentium Ununseptium Ununoctium Ce 58 Pr 59 Nd 60 Pm 61 Sm 62 Eu 63 Gd 64 Tb 65 Dy 66 Ho 67 Er 68 Tm 69 Yb 70 Lu 71 6 Cerium Praseodymium Neodymium Promethium Samarium Europium Gadolinium Terbium Dysprosium Holmium Erbium Thulium Ytterbium Lutetium Th 90 Pa 91 U 92 Np 93 Pu 94 Am 95 Cm 96 Bk 97 Cf 98 Es 99 Fm 100 Md 101 No 102 Lr 103 7 Thorium Protactinium Uranium Neptunium Plutonium Americium Curium Berkelium Californium Einsteinium Fermium Mendelevium Nobelium Lawrencium Non-metals N ot a literal “gro u p” • Diverse properties; very little commonality • Outer electrons in the p orbitals Non-metals Prof Mokeur's Periodic Table IA H1 VIIIA He 2 1 Hydrogen IIA Li 3 Be 4 H1 B5 Lithium Beryllium Hydrogen Boron IIIA IVA C6 VA N7 VIA VIIA F9 Ne 10 Carbon Nitrogen Oxygen Fluorine Neon P 15 S 16 Cl 17 Ar 18 Phosphorus Sulfur Chlorine O8 Helium 2 Na 11 Mg 12 Al 13 Si 14 3 Sodium K 19 Magnesium IIIB IVB VB Ca 20 Sc 21 Ti 22 V 23 VIB VIIB VIII IB IIB Aluminum Silicon Cr 24 Mn 25 Fe 26 Co 27 Ni 28 Cu 29 Zn 30 Ga 31 Ge 32 As 33 Argon Se 34 Br 35 Kr 36 4 Potassium Calcium Rb 37 Sr 38 Scandium Y 39 Titanium Vanadium Chromium Manganese Iron Strontium Yttrium Nickel Copper Zinc Gallium Germanium Arsenic Selenium Zr 40 Nb 41 Mo 42 Tc 43 Ru 44 Rh 45 Pd 46 Ag 47 Cd 48 In 49 Sn 50 Sb 51 Te 52 5 Rubidium Cobalt Zirconium Niobium 4,3 Molybdenum Technetium Ruthenium Rhodium Palladium Silver Cadmium 4,2 -4 Tin Indium 5,3 -3 Antimony Tellurium Bromine Krypton I 53 Xe 54 Xenon Iodine Cs 55 Ba 56 La 57 Hf 72 Ta 73 W 74 Re 75 Os 76 Ir 77 Pt 78 Au 79 Hg 80 Tl 81 Pb 82 Bi 83 Po 84 At 85 Rn 86 Cesium Platinum 6 Barium Lanthanum Hafnium Tantalum Tungsten Rhenium Osmium Iridium Gold Mercury Thallium Lead Bismuth Polonium Fr 87 Ra 88 Ac 89 Rf 104 Db 105 Sg 106 Bh 107 Hs 108 Mt 109 Ds 110 Rg 111 Cn 112 Uut 113 114 Uup 115 Astatine Radon 116 Uus 117 Uuo 118 7 Francium Radium Actinium Rutherfordium Dubnium Seaborgium Bohrium Hassium Meitnerium Darmstadtium Roentgenium Copernicium Ununtrium Ununpentium Ununseptium Ununoctium Ce 58 Pr 59 Nd 60 Pm 61 Sm 62 Eu 63 Gd 64 Tb 65 Dy 66 Ho 67 Er 68 Tm 69 Yb 70 Lu 71 6 Cerium Praseodymium Neodymium Promethium Samarium Europium Gadolinium Terbium Dysprosium Holmium Erbium Thulium Ytterbium Lutetium Th 90 Pa 91 U 92 Np 93 Pu 94 Am 95 Cm 96 Bk 97 Cf 98 Es 99 Fm 100 Md 101 No 102 Lr 103 7 Thorium Protactinium Uranium Neptunium Plutonium Americium Curium Berkelium Californium Einsteinium Fermium Mendelevium Nobelium Lawrencium Halogens • 7 electrons in their outer orbitals • Highly reactive • Form salts with metals Halogens Prof Mokeur's Periodic Table IA H1 VIIIA He 2 1 Hydrogen IIA Li 3 Be 4 H1 B5 Lithium Beryllium Hydrogen Boron IIIA IVA C6 VA N7 VIA VIIA F9 Ne 10 Carbon Nitrogen Oxygen Fluorine Neon P 15 S 16 Cl 17 Ar 18 Phosphorus Sulfur Chlorine O8 Helium 2 Na 11 Mg 12 Al 13 Si 14 3 Sodium K 19 Magnesium IIIB IVB VB Ca 20 Sc 21 Ti 22 V 23 VIB VIIB VIII IB IIB Aluminum Silicon Cr 24 Mn 25 Fe 26 Co 27 Ni 28 Cu 29 Zn 30 Ga 31 Ge 32 As 33 Argon Se 34 Br 35 Kr 36 4 Potassium Calcium Rb 37 Sr 38 Scandium Y 39 Titanium Vanadium Chromium Manganese Iron Strontium Yttrium Nickel Copper Zinc Gallium Germanium Arsenic Selenium Zr 40 Nb 41 Mo 42 Tc 43 Ru 44 Rh 45 Pd 46 Ag 47 Cd 48 In 49 Sn 50 Sb 51 Te 52 5 Rubidium Cobalt Zirconium Niobium 4,3 Molybdenum Technetium Ruthenium Rhodium Palladium Silver Cadmium 4,2 -4 Tin Indium 5,3 -3 Antimony Tellurium Bromine Krypton I 53 Xe 54 Xenon Iodine Cs 55 Ba 56 La 57 Hf 72 Ta 73 W 74 Re 75 Os 76 Ir 77 Pt 78 Au 79 Hg 80 Tl 81 Pb 82 Bi 83 Po 84 At 85 Rn 86 Cesium Platinum 6 Barium Lanthanum Hafnium Tantalum Tungsten Rhenium Osmium Iridium Gold Mercury Thallium Lead Bismuth Polonium Fr 87 Ra 88 Ac 89 Rf 104 Db 105 Sg 106 Bh 107 Hs 108 Mt 109 Ds 110 Rg 111 Cn 112 Uut 113 114 Uup 115 Astatine Radon 116 Uus 117 Uuo 118 7 Francium Radium Actinium Rutherfordium Dubnium Seaborgium Bohrium Hassium Meitnerium Darmstadtium Roentgenium Copernicium Ununtrium Ununpentium Ununseptium Ununoctium Ce 58 Pr 59 Nd 60 Pm 61 Sm 62 Eu 63 Gd 64 Tb 65 Dy 66 Ho 67 Er 68 Tm 69 Yb 70 Lu 71 6 Cerium Praseodymium Neodymium Promethium Samarium Europium Gadolinium Terbium Dysprosium Holmium Erbium Thulium Ytterbium Lutetium Th 90 Pa 91 U 92 Np 93 Pu 94 Am 95 Cm 96 Bk 97 Cf 98 Es 99 Fm 100 Md 101 No 102 Lr 103 7 Thorium Protactinium Uranium Neptunium Plutonium Americium Curium Berkelium Californium Einsteinium Fermium Mendelevium Nobelium Lawrencium Noble Gases • 8 electrons in the outer energy level; outer energy level filled • Non-reactive, do not react readily with any other elements • All are gas at room temperature and well below Alkaline Earth Metals (IIA) Prof Mokeur's Periodic Table IA H1 VIIIA He 2 1 Hydrogen IIA Li 3 Be 4 H1 B5 Lithium Beryllium Hydrogen Boron IIIA IVA C6 VA N7 VIA VIIA F9 Ne 10 Carbon Nitrogen Oxygen Fluorine Neon P 15 S 16 Cl 17 Ar 18 Phosphorus Sulfur Chlorine O8 Helium 2 Na 11 Mg 12 Al 13 Si 14 3 Sodium K 19 Magnesium IIIB IVB VB Ca 20 Sc 21 Ti 22 V 23 VIB VIIB VIII IB IIB Aluminum Silicon Cr 24 Mn 25 Fe 26 Co 27 Ni 28 Cu 29 Zn 30 Ga 31 Ge 32 As 33 Argon Se 34 Br 35 Kr 36 4 Potassium Calcium Rb 37 Sr 38 Scandium Y 39 Titanium Vanadium Chromium Manganese Iron Strontium Yttrium Nickel Copper Zinc Gallium Germanium Arsenic Selenium Zr 40 Nb 41 Mo 42 Tc 43 Ru 44 Rh 45 Pd 46 Ag 47 Cd 48 In 49 Sn 50 Sb 51 Te 52 5 Rubidium Cobalt Zirconium Niobium 4,3 Molybdenum Technetium Ruthenium Rhodium Palladium Silver Cadmium 4,2 -4 Tin Indium 5,3 -3 Antimony Tellurium Bromine Krypton I 53 Xe 54 Xenon Iodine Cs 55 Ba 56 La 57 Hf 72 Ta 73 W 74 Re 75 Os 76 Ir 77 Pt 78 Au 79 Hg 80 Tl 81 Pb 82 Bi 83 Po 84 At 85 Rn 86 Cesium Platinum 6 Barium Lanthanum Hafnium Tantalum Tungsten Rhenium Osmium Iridium Gold Mercury Thallium Lead Bismuth Polonium Fr 87 Ra 88 Ac 89 Rf 104 Db 105 Sg 106 Bh 107 Hs 108 Mt 109 Ds 110 Rg 111 Cn 112 Uut 113 114 Uup 115 Astatine Radon 116 Uus 117 Uuo 118 7 Francium Radium Actinium Rutherfordium Dubnium Seaborgium Bohrium Hassium Meitnerium Darmstadtium Roentgenium Copernicium Ununtrium Ununpentium Ununseptium Ununoctium Ce 58 Pr 59 Nd 60 Pm 61 Sm 62 Eu 63 Gd 64 Tb 65 Dy 66 Ho 67 Er 68 Tm 69 Yb 70 Lu 71 6 Cerium Praseodymium Neodymium Promethium Samarium Europium Gadolinium Terbium Dysprosium Holmium Erbium Thulium Ytterbium Lutetium Th 90 Pa 91 U 92 Np 93 Pu 94 Am 95 Cm 96 Bk 97 Cf 98 Es 99 Fm 100 Md 101 No 102 Lr 103 7 Thorium Protactinium Uranium Neptunium Plutonium Americium Curium Berkelium Californium Einsteinium Fermium Mendelevium Nobelium Lawrencium Actinides and Lanthanides • Outer electrons are in f block • Rare elements that can be superconductors, semiconductors, very radioactive, etc.