Physical Chemistry 1 - Sultan Qaboos University
advertisement

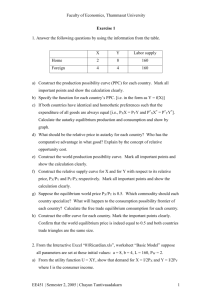
Course Outline: CHEM 3333 Physical ChemistryII Sultan Qaboos University College of Science - Department of Chemistry Physical Chemistry I (CHEM-3333) Course Outline General information Credits and contact hours: 3 credits; 60 contact hours Prerequisite: CHEM2102 + MATH2107 + PHYS2101 Co-requisite: CHEM3335 Keywords: Thermodynamics and Laws of Thermodynamics, Material Equilibrium, Reaction Equilibrium, One-Component Phase Equilibrium and Chemical Kinetics. Textbook and references: Physical Chemistry, LEVINE, 6th edition, McGraw-Hill, 2009. Assessment: Lecture Quizzes Mid-term Tests Final Exam Grades: The following grades are TENTATIVE, the cutoffs can be lowered but they will not be raised. A ≥ 84, A- ≥ 80, B+ ≥ 77, B ≥ 73, B- ≥ 70, C+ ≥ 67, C ≥ 63, C- ≥ 59, D+ ≥ 55, D ≥ 50, F<50 10% 40% 50% Description This is the first of a two-part survey of physical chemistry. Topics are selected from the broad themes of the discipline: thermodynamics, chemical kinetics and their applications to chemistry. Physical chemistry uses differential and integral calculus extensively. Calculus 2101 was therefore required as a prerequisite. The co-requisite laboratory component (CHEM3335) is aimed to reinforce the subject matter learnt in lectures. Objectives This course seeks to: ● Apply thermodynamic and kinetic principals to chemical reactions ● Grasp the fundamental difference between thermodynamic and kinetic approaches in chemistry ● Apply the concepts and principles to solve practical problems. Learning Outcomes Knowledge On successful completion of this course, students will be able to: ● calculate P (or V or T) of an ideal gas or ideal gas mixture, perform calculations involving differentiation (ordinary and partial derivations), use of and to find volume changes produced by changes in T or P. ● use the first law of thermodynamics to calculate heat (q), work (w), change of the internal energy (∆U) and enthalpy (∆H) for different thermodynamic processes (isobaric, isothermal, adiabatic,..). ● use the second law of thermodynamics to calculate entropy change (∆S) for different reversible processes (phase change, constant-pressure heating, change of state of a perfect gas, mixing perfect gases at constant T and P) 1 Course Outline: CHEM 3333 Physical ChemistryII ● calculate ∆U, ∆H, and ∆S for changes in system temperature and pressure as well as ∆G for isothermal processes ● Calculate C P – C V , (∂U/∂V) T , (∂S/∂T) P , (∂S/∂P) T , etc.., from readily measured properties (C P , α, κ). ● determine ∆Ho of a reaction using Hess’s Law, calculate ∆ c U from adiabatic bomb calorimeter data, calculate ∆Ho from ∆Uo and vice versa, calculate the standard thermodynamic functions (∆Ho, ∆So, and ∆Go) for chemical reactions from tabulated ∆ f Ho, So m , and ∆ f Go data, determine ∆Ho (or ∆So) at one temperature from ∆Ho (or ∆So) at another temperature and Co P,m (T) data ● calculate the equilibrium constant Ko P and ∆Go from the observed equilibrium composition, calculate Ko P from ∆Go using ∆Go = – RT ln Ko P , calculate the equilibrium composition from Ko P for constant-Tand-P or constant-T-and-Vconditions, calculate Ko P at different temperatures using Van’t Hoff equation. ● use the phase rule to find the number of degree of freedom f, use d lnP/dT = ∆H m /RT2 and a vaporpressure data to find ∆ vap H m or ∆ sub H m of a pure substance, use d lnP/dT = ∆H m /RT2 and the vapor pressure at one temperature to find the vapor pressure at another temperature, use d lnP/dT = ∆H m /RT2 to find the boiling point at a given temperature from the normal boiling point, use the Clapeyron equation to find the change in melting point with pressure. ● use nonideal equations of such as the van der Walls, the Redlich-Kwong, and the virial components to calculate P or V of a pure gas or a gas mixture, and calculate constants in the van der Walls equation from critical-point data ● calculate the amounts of reactants and products present at a given time from the integrated rate law and the initial composition, determine the reaction orders and the rate constant from kinetic data, find the mechanism for certain chemical reactions, use Arrhenius equation in order to calculate A and E a from k-versus-T data or to calculate k(T 2 ) from k(T 1 ). Skills and Attitudes On successful completion of this course, students will be expected to: ● acquire critical thinking ● manage their time and organize their work efficiently ● be convinced that understanding, rather than mindless memorization, is the key to learning in physical chemistry ● acquire problem-solving skills Instructional Methods: Lectures • • • Students should be punctual in the class. The lectures will be primarily based on the material in the textbook. Other information is provided through e-learning tools. Course Assessment: Lecture Quizzes There will be a number of random quizzes directly after completion of each chapter for a short time which counts 10% toward the final course mark. Tests There will be at least two mid-term tests, each of 75 minutes duration. These tests will count 40% toward the final course mark. The material covered for these tests will be announced before the each test. 2 Course Outline: CHEM 3333 Physical ChemistryII Final Exam The final exam is comprehensive, and will be of three hours duration. The timetable of the final exam will be announced by the University Timetabling Office. This exam will count 50% towards the final course mark. Course Contents: Chapter 1: Thermodynamics ● Calculation of P (or V or T) of an ideal gas or ideal gas mixture using PV = nRT ● Calculation of the molar mass of an ideal gas using PV = nRT and n = m/M ● Calculation of the density of an ideal gas ● Calculations involving partial pressures ● Use of α and κ to find volume changes produced by changes in T or P ● Differentiation and partial differentiation of functions ● Indefinite and definite integration of functions Chapter 2: The First Law of Thermodynamics Calculation of q, w, ∆U, and ∆H for ● Phase changes (for example, melting) ● Heating a substance at constant pressure ● Heating at constant volume ● An isothermal reversible process in a perfect gas ● An adiabatic reversible process in a perfect gas with C V constant ● A constant-pressure reversible process in a perfect gas ● A constant-volume reversible process in a perfect gas ● A constant-temperature reversible process in a perfect gas Chapter 3: The Second Law of Thermodynamics ● Calculation of ∆S for a reversible process using dS = d qrev dT ● Calculation of ∆S for an irreversible process by finding a reversible path between the initial and final states ● Calculation of ∆S for a reversible phase change using ∆S = DH/T ● Calculation of ∆S for constant-pressure heating using dS = dqrev/T = (Cp/T)dT ● Calculation of ∆S for for a change of state of a perfect gas ● Calculation of ∆S for mixing perfect gases at constant T and P Chapter 4: Material Equilibrium ● Calculation of ∆U, ∆H, and ∆S for changes in system temperature and pressure and calculation of ∆G for isothermal processes ● Calculation of C P – C V , (∂U/∂V) T , (∂S/∂T) P , (∂S/∂P) T , etc.., from readily measured properties (C P , α, κ). Chapter 5: Standard Thermodynamic Functions of Reactions ● Determination of ∆Ho of a reaction by combining ∆Ho values from reactions (Hess’s Law) ● Calculation of ∆ r U from adiabatic bomb calorimeter data ● Calculation of ∆Ho from ∆Uo and vice versa ● Calculation of ∆Ho, ∆So, and ∆Go of chemical reactions from tabulated ∆ f Ho, So m , and ∆ f Go data ● Determination of ∆Ho (or ∆So) at one temperature from ∆Ho (or ∆So) at another temperature and Co P,m (T) data Chapter 6: Reaction Equilibrium in Ideal Gas Mixtures ● Calculation of Ko P and ∆Go from the observed equilibrium composition ● Calculation of Ko P from ∆Go using ∆Go = – RT ln Ko P 3 Course Outline: CHEM 3333 Physical ChemistryII ● Calculation of the equilibrium composition from Ko P and the initial for constant-T-and-P or constantT-and-V conditions ● Calculation of Ko P at T 2 from Ko P at T 1 using dln Ko P /dT = ∆Ho/RT2 ● Calculation of ∆Ho, ∆Go, and ∆So from Ko P versus T data using ∆Go = - RT ln Ko P to get ∆Go, dln Ko P /dT = ∆Ho/RT2 to get ∆Ho, and ∆Go = ∆Ho – T∆So to get ∆So Chapter 7: One-Component Phase Equilibrium ● Use the phase rule to find the number of degree of freedom f ● Use d lnP/dT = ∆H m /RT2 and a vapor-pressure data to find ∆ vap H m or ∆ sub H m of a pure substance ● Use d lnP/dT = ∆H m /RT2 and the vapor pressure at one temperature to find the vapor pressure at another temperature ● Use d lnP/dT = ∆H m /RT2 to find the boiling point at a given temperature from the normal boiling point ● Use the Clapeyron equation to find the change in melting point with pressure Chapter 8: Real Gases ● Use of nonideal equations of such as the van der Walls, the Redlich-Kwong, and the virial components to calculate P or V of a pure gas or a gas mixture ● Calculation of constants in the van der Walls equation from critical-point data Chapter 9: Solutions ● Solution composition, partial molar quantities, mixing quantities ● Determination of partial molar quantities ● Ideal solution, thermodynamics properties of ideal solution Chapter 10: Non-ideal solutions ● Activities and activity coefficients, excess functions, determination of activities ● Determination of electrolyte activity coefficients ● The Debye-Huckel theory of electrolyte solutions and non-ideal mixtures Chapter 16: Reaction Kinetics ● Calculation of the amounts of reactants and products present at a given time from the integrated rate law and the initial composition ● Determination of reaction orders from kinetic data ● Determination of the rate constant from kinetic data ● Use of Arrhenius equation to calculate A and E a from k-versus-T data or to calculate k(T 2 ) from k(T 1 ) and A and E a 4