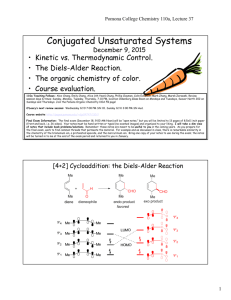
genetic engineering of chlorella zofingiensis for
advertisement

Title Author(s) Genetic engineering of Chlorella zofingiensis for enhanced astaxanthinbiosynthesis and assessment of the algal oil for biodiesel production Liu, Jin; 刘进 Citation Issued Date URL Rights 2010 http://hdl.handle.net/10722/131813 The author retains all proprietary rights, (such as patent rights) and the right to use in future works. GENETIC ENGINEERING OF CHLORELLA ZOFINGIENSIS FOR ENHANCED ASTAXANTHIN BIOSYNTHESIS AND ASSESSMENT OF THE ALGAL OIL FOR BIODIESEL PRODUCTION by LIU Jin B.Sc. (Sun Yat-sen University, P.R. China) M.Sc. (Sun Yat-sen University, P.R. China) A thesis submitted in fulfillment of the requirements for the degree of Doctor of Philosophy at The University of Hong Kong July 2010 Abstract of thesis entitled GENETIC ENGINEERING OF CHLORELLA ZOFINGIENSIS FOR ENHANCED ASTAXANTHIN BIOSYNTHESIS AND ASSESSMENT OF THE ALGAL OIL FOR BIODIESEL PRODUCTION Submitted by LIU Jin for the degree of Doctor of Philosophy at The University of Hong Kong in July 2010 Chlorella zofingiensis is an important microalga for astaxanthin (a high-value ketocarotenoid) and biodiesel. In this thesis, C. zofingiensis was genetically engineered for enhanced astaxanthin production. In addition, the potential of the alga was assessed for biodiesel production. C. zofingiensis was found to be very sensitive to the herbicide norflurazon which specifically inhibits the activity of phytoene desaturase (PDS), a rate-limiting enzyme in carotenoid biosynthesis. Mutated PDS genes resistant to norflurazon were proposed to be used as dominant selectable markers for genetic engineering of algae. To address this issue, C. zofingiensis mutants resistant to norflurazon were made by treating the algal cells with chemical mutagens and screening with norflurazon. One mutant E17 produced 54% more astaxanthin than its wild type (WT). A point mutation (C to T) was found to occur in its PDS gene, leading to an amino acid change (leucine to phenylalanine) at position 516. The mutated PDS exhibited 30-fold higher resistance to norflurazon and 30% greater desaturation activity than its WT one. The PDS-L516F gene was delivered into WT C. zofingiensis via the biolistic approach. Transgenic C. zofingiensis showed norflurazon resistance and accumulated up to 46.3% more astaxanthin than WT, suggesting that the mutated PDS gene could serve as a selectable marker for genetic engineering of C. zofingiensis and concurrently for enhanced production of astaxanthin. C. zofingiensis cells cultivated under different growth modes showed differential lipid and fatty acid profiles. Compared with photoautotrophic cells, a 900% increase in lipid yield was achieved in heterotrophic cells. Furthermore heterotrophic cells accumulated predominantly neutral lipids (NL) that accounted for 79.5% of total lipids, with 88.7% of NL being triacylglycerol (TAG); whereas photoautotrophic cells contained mainly glycolipids (GL) and phospholipids (PL). Together with the much higher content of oleic acid (C18:1), oils from heterotrophic C. zofingiensis appear to be more feasible for biodiesel production. A number of nutritional and environmental factors were found to influence the accumulation of fatty acids in C. zofingiensis. The optimal nutritional and environmental conditions for total fatty acid (TFA) production by C. zofingiensis were 5 mM nitrate, 5 mM phosphate, 25-100 μM ferrous ion, temperature 25 °C and pH 6.5. High light and glucose were revealed to up-regulate the expression of both biotin carboxylase (BC) and stearoyl ACP desaturase (SAD) genes that are involved in fatty acid biosynthesis and therefore enhanced the accumulation of TFA including oleic acid. Unlike high light or glucose, salt stress, however, could only trigger the up-regulation of SAD gene; accordingly, the TFA content was slightly affected while the biosynthesis of oleic acid was promoted. In conclusion, a selectable marker and transgenic C. zofingiensis system have been developed for enhanced production of astaxanthin. C. zofingiensis was shown to have great potential for biodiesel production. The study provides novel information on both carotenoid and fatty acid biosynthesis, which is important for the exploitation of the alga as a source of natural astaxanthin as well as biodiesel. GENETIC ENGINEERING OF CHLORELLA ZOFINGIENSIS FOR ENHANCED ASTAXANTHIN BIOSYNTHESIS AND ASSESSMENT OF THE ALGAL OIL FOR BIODIESEL PRODUCTION by LIU Jin B.Sc. (Sun Yat-sen University, P.R. China) M.Sc. (Sun Yat-sen University, P.R. China) A thesis submitted in fulfillment of the requirements for the degree of Doctor of Philosophy at The University of Hong Kong July 2010 DECLARATION I declare that this thesis represents my own work, except where due acknowledgement is made, and that it has not been previously included in a thesis, dissertation or report submitted to this University or to any other institution for a degree, diploma or other qualification. Signed…………………………………..………… LIU Jin i ACKNOWLEDGEMENTS First and foremost, I would like to thank my supervisor Prof. Steven Feng Chen for his helpful guidance, invaluable advice and continuous supports for my research and life during the whole PhD study. I also thank Dr. Junchao Huang for his great help on my works and constructive suggestions on my manuscripts and thesis. His patience and kindness make me enjoy the research. I am greatful to Prof. Gerhard Sandmann of Geothe University and Dr. Yue Jiang of Hong Kong Baptist University for their advices on my manuscripts. My thanks also extend to Dr. Mingfu Wang, Dr. Clive S.C. Lo and Dr. W.K. Yip for their kind supply of laboratory apparatus. Many thanks are given to my lab-mates and collegues for their kind assistance and memorable friendship: Dr. Keith K.W. Fan, Mr. Zheng Sun, Ms. Yujuan Zhong, Dr. Yantao Li, Dr. Guangquan Chen, Dr. Huabing Li, Dr. Yizhong Cai, Ms. Jieqiong Huangfu, Ms. Lily R.L. Suen, Dr. Ni Sun, Dr. Bevin S.Y. Ho, Mr. Dennis C.C. Wong, Dr. Yan Wang. Finally, I would like to express my thanks to my parents, my young brother and his wife, and my lovely nephew for their infinite physical and emotional support and encouragement. To them, my appreciation never ends. ii PUBLICATIONS Journal papers Liu J, Huang J, Fan K-W, Jiang Y, Zhong Y, Sun Z, Chen F (2010) Production potential of Chlorella zofingienesis as a feedstock for biodiesel. Bioresource Technology 101: 8658-8663 Liu J, Huang J, Sun Z, Zhong Y, Jiang Y, Chen F (2010) Differential lipid and fatty acid profiles of photoautotrophic and heterotrophic Chlorella zofingiensis: Assessment of algal oils for biodiesel production. Bioresource Technology, in press Liu J, Zhong Y, Sun Z, Huang J, Sandmann G, Chen F (2010) One amino acid substitution in phytoene desaturase makes Chlorella zofingiensis resistant to norflurazon and enhances the biosynthesis of astaxanthin. Planta 232: 61-67 Sun Z, Peng X, Liu J, Fan K-W, Wang M, Chen F (2010) Inhibitory effects of microalgal extracts on the formation of advanced glycation endproducts (AGEs). Food Chemistry 120: 261-267 Huang JC, Liu J*, Li YT, Chen F (2008) Isolation and characterization of the phytoene desaturase gene as a potential selective marker for genetic engineering of the astaxanthin-producing green alga Chlorella zofingiensis (Chlorophyta). Journal of Phycology 44: 684-690 *Co-first author Conference papers Liu J, Huang J, Chen F (2009) Metabolic engineering of Chlorella zofingiensis (Chlorophyta) for enhanced biosynthesis of astaxanthin. FEBS Journal 276: S283-S283 Liu J, Huang J, Sandmann G, Chen F (2008) Metabolic engineering for enhanced astaxanthin biosynthesis in Chlorella zofingiensis (chlorophyta). Journal of Biotechnology 136: S572-S572 iii CONTENTS DECLARATION ................................................................................................. i ACKNOWLEDGEMENTS ................................................................................. ii PUBLICATIONS ............................................................................................... iii CONTENTS........................................................................................................iv LIST OF ABBREVIATIONS ..............................................................................xi PART I. LITERATURE REVIEW AND RESEARCH AIM ................................. 1 Chapter 1. Literature review and research aim ............................................ 2 1.1 Introduction ............................................................................................ 2 1.2 Astaxanthin ............................................................................................. 3 1.2.1 Biochemical properties of astaxanthin............................................... 3 1.2.2 Applications of astaxanthin ............................................................... 6 1.2.2.1 Astaxanthin as a coloring agent .................................................. 6 1.2.2.2 Astaxanthin as an antioxidant ..................................................... 7 1.2.3 Potential sources of astaxanthin ........................................................ 9 1.2.3.1 Synthetic astaxanthin .................................................................10 1.2.3.2 Astaxanthin from crustacean by-products ..................................10 1.2.3.3 Astaxanthin from yeast .............................................................. 11 1.2.3.4 Astaxanthin from microalgae .....................................................12 1.2.3.5 Astaxanthin from transgenic plants ............................................14 1.2.3.6 Natural astaxanthin versus synthetic astaxanthin........................14 1.2.4 Genetic modification for enhanced astaxanthin accumulation ..........15 1.2.4.1 Mutagenesis ..............................................................................15 1.2.4.2 Genetic engineering of the carotenoid biosynthetic pathway ......16 1.3 Biodiesel................................................................................................25 1.3.1 Introduction .....................................................................................25 1.3.2 Biodiesel production through transesterification ..............................28 1.3.3 Biodiesel feedstocks ........................................................................32 iv 1.3.3.1 Plant oils ...................................................................................32 1.3.3.2 Animal fats and waste oils .........................................................34 1.3.3.3 Algal oils ...................................................................................34 1.3.4 Potential and prospect of microalgal biodiesel .................................35 1.3.5 Lipid metabolism in microalgae .......................................................42 1.3.5.1 Fatty acid/lipid biosynthesis ......................................................42 1.3.5.2 Factors affecting lipid accumulation and fatty acid composition 45 1.4 The green alga Chlorella zofingiensis .....................................................48 1.4.1 Pigment profiles ..............................................................................49 1.4.2 Astaxanthin biosynthesis ..................................................................53 1.4.3 Lipid and fatty acid profiles .............................................................55 1.5 Research aim .........................................................................................55 PART II. GENETIC ENGINEERING OF CHLORELLA ZOFINGIENSIS FOR ENHANCED ASTAXANTHIN PRODUCTION ................................................57 Chapter 2. Isolation and characterization of the phytoene desaturase gene from Chlorella zofingiensis ............................................................................58 2.1 Abstract .................................................................................................58 2.2 Introduction ...........................................................................................58 2.3 Materials and methods ...........................................................................60 2.3.1 Algal strain and culture conditions ...................................................60 2.3.2 Genomic DNA and RNA isolation ...................................................60 2.3.3 Cloning of PDS cDNA and its corresponding gene ..........................61 2.3.4 Functional analysis of PDS cDNA ...................................................61 2.3.5 RT-PCR assay ..................................................................................62 2.3.6 Preparation of enzyme and substrate ................................................62 2.3.7 In vitro PDS assay ...........................................................................63 2.3.8 Pigment analysis ..............................................................................63 2.4 Results and discussion ...........................................................................64 2.4.1 Cloning of C. zofingiensis PDS gene................................................64 v 2.4.2 Functional analysis of C. zofingiensis PDS cDNA in E. coli ............66 2.4.3 C. zofingiensis PDS gene is up-regulated by high light and glucose .70 2.4.4 PDS-L516R is resistant to the herbicide norflurazon ........................71 Chapter 3. Isolation and characterization of Chlorella zofingiensis mutants with enhanced biosynthesis of astaxanthin ..................................................75 3.1 Abstract .................................................................................................75 3.2 Introduction ...........................................................................................76 3.3 Methods and materials ...........................................................................76 3.3.1 Algal strain and culture conditions ...................................................76 3.3.2 Mutagenesis.....................................................................................77 3.3.4 Isolation of Chlorella mutants ..........................................................77 3.3.5 Cell dry weight determination ..........................................................77 3.3.6 Astaxanthin induction ......................................................................78 3.3.7 Extraction and analysis of pigments .................................................78 3.3.8 RNA isolation and RT-PCR assay ....................................................78 3.4 Results ...................................................................................................79 3.4.1 Carotenoid biosynthesis blocked by norflurazon ..............................79 3.4.2 Isolation of Chlorella mutants resistant to norflurazon .....................80 3.4.3 Growth and astaxanthin accumulation of mutants and WT ...............82 3.4.4 Accumulation of TC and astaxanthin induced by glucose .................84 3.4.5 Expression analysis of carotenogenic genes .....................................86 3.5 Discussion .............................................................................................86 Chpater 4. Molecular characterization of the Chlorella zofingiensis mutant E17 .................................................................................................................90 4.1 Abstract .................................................................................................90 4.2 Introduction ...........................................................................................91 4.3 Methods and materials ...........................................................................91 4.3.1 Algal strain and culture conditions ...................................................91 4.3.2 Cell dry weight determination ..........................................................92 4.3.3 Extraction and analysis of pigments .................................................92 vi 4.3.4 Chlorophyll fluorescence measurement............................................92 4.3.5 RNA isolation and RT-PCR assay ....................................................92 4.3.6 PDS expression in E. coli.................................................................92 4.3.7 Enzyme and substrate preparation and in vitro PDS assay ................93 4.4 Results ...................................................................................................93 4.4.1 The growth and carotenogensis of E17.............................................93 4.4.2 Enhanced production of TC and astaxanthin by E17 under high light stress or glucose induction ........................................................................95 4.4.3 Characterization of E17 PDS ...........................................................98 4.4.4 Transcription analysis of PDS, BKT and CHYb genes .................... 100 4.5 Discussion ........................................................................................... 101 Chapter 5. Transformation of Chlorella zofingiensis with a phytoene desaturase gene for enhanced astaxanthin accumulation .......................... 104 5.1 Abstract ............................................................................................... 104 5.2 Introduction ......................................................................................... 104 5.3 Materials and methods ......................................................................... 106 5.3.1 Algal strain and culture conditions ................................................. 106 5.3.2 Construction of the transformation vector pBlue-PDS-L516F and transformation protocol .......................................................................... 107 5.3.3 Genomic DNA and RNA isolation ................................................. 108 5.3.4 PCR determination of transformants .............................................. 108 5.3.5 Cell dry weight determination ........................................................ 108 5.3.6 Extraction and analysis of pigments ............................................... 109 5.3.7 RT-PCR assay ................................................................................ 109 5.4 Results ................................................................................................. 109 5.4.1 Analysis of C. zofingiensis transformants ....................................... 109 5.4.2 Resistance of transformants to norflurazon .................................... 111 5.4.3 Enhanced biosynthesis of astaxanthin in P6 induced by glucose..... 113 5.4.4 Transcription analysis of carotenogenic genes ................................ 115 5.5 Discussion ........................................................................................... 116 vii PART III. POTENTIAL ASSESSMENT OF CHLORELLA ZOFINGIENSIS AS A BIODIESEL FEEDSTOCK.............................................................................. 119 Chapter 6. Differential lipid and fatty acid profiles of photoautotrophic and heterotrophic Chlorella zofingiensis: assessment of algal oils for biodiesel production ................................................................................................... 120 6.1 Abstract ............................................................................................... 120 6.2 Introduction ......................................................................................... 121 6.3 Methods and materials ......................................................................... 122 6.3.1 Algal strain and culture conditions ................................................. 122 6.3.2 Determination of glucose concentration, nitrate concentration, cell dry weight and specific growth rate .............................................................. 123 6.3.3 Lipid extraction and analysis ......................................................... 123 6.3.4 Fatty acid analysis ......................................................................... 124 6.4.5 Calculation of iodine value ............................................................ 124 6.5 Results ................................................................................................. 125 6.5.1 Growth characteristics and fatty acid accumulation ........................ 125 6.5.2 Lipid class composition ................................................................. 127 6.5.3 Fatty acid composition of individual lipid classes .......................... 129 6.6 Discussion ........................................................................................... 131 Chapter 7. Sugar-based growth and lipid accumulation for oil production in heterotrophic Chlorella zofingienesis...................................................... 134 7.1 Abstract ............................................................................................... 134 7.2 Introduction ......................................................................................... 135 7.3 Methods and materials ......................................................................... 135 7.3.1 Algal strain and culture conditions ................................................. 135 7.3.2 Pretreatment of molasses ............................................................... 136 7.3.3 Batch and fed-batch culture ........................................................... 136 7.3.4 Determination of glucose and nitrate concentration, cell dry weight and specific growth rate.......................................................................... 137 viii 7.3.5 Lipid extraction and analysis ......................................................... 137 7.3.6 Fatty acid analysis ......................................................................... 137 7.3.7 RNA isolation and RT-PCR assay .................................................. 137 7.4 Results ................................................................................................. 138 7.4.1 Heterotrophic growth and lipid production of C. zofingiensis with various carbon sources ........................................................................... 138 7.4.2 Fatty acid profiles of dark-grown C. zofingiensis cultures .............. 140 7.4.3 Sugars up-regulate the transcription of BC and SAD genes of C. zofingiensis ............................................................................................ 142 7.4.4 Fed-batch fermentation enhances lipid production by C. zofingiensis ............................................................................................................... 143 7.4.5 Assessment of cane molasses as the carbon source for lipid production by C. zofingiensis ................................................................................... 145 7.5 Discussion ........................................................................................... 146 Chapter 8. Production of fatty acids by heterotrophic Chlorella zofingiensis: influences of nutritional and environmental factors .................................. 149 8.1 Abstract ............................................................................................... 149 8.2 Introduction ......................................................................................... 150 8.3 Methods and materials ......................................................................... 150 8.3.1 Algal strain and culture conditions ................................................. 150 8.3.2 Determination of cell dry weight.................................................... 151 8.3.3 Fatty acid analysis ......................................................................... 151 8.4 Results and discussion ......................................................................... 151 8.4.1 Nitrate ........................................................................................... 151 8.4.2 Phosphate ...................................................................................... 154 8.4.3 Ferrous ion .................................................................................... 155 8.4.4 Cultivation temperature ................................................................. 156 8.4.5 Initial pH of culture medium .......................................................... 157 Chapter 9. Isolation and characterization of biotin carboxylase gene and stearoyl ACP desaturase gene from Chlorella zofingiensis ........................ 159 ix 9.1 Abstract ............................................................................................... 159 9.2 Introduction ......................................................................................... 160 9.3 Methods and materials ......................................................................... 160 9.3.1 Algal strain and culture conditions ................................................. 160 9.3.2 Genomic DNA and RNA isolation ................................................. 161 9.3.3 Cloning of BC cDNA, SAD cDNA and their corresponding genes .. 161 9.3.4 RT-PCR assay ................................................................................ 163 9.3.5 Fatty acid analysis ......................................................................... 163 9.4 Results ................................................................................................. 163 9.4.1 Cloning and characterization of the BC and SAD gene from C. zofingiensis ............................................................................................ 163 9.4.2 High light irradiation up-regulates the transcripts of BC and SAD and enhances the biosynthesis of TFA and oleic acid ..................................... 167 9.4.3 Salt stress induces the up-regulation of SAD gene and the accumulation of oleic acid ...................................................................... 169 9.4.5 Glucose triggers the up-regulation of BC and SAD genes and induces the enhanced biosynthesis of TFA and oleic acid .................................... 170 9.5 Discussion ........................................................................................... 171 PART IV. RESEARCH SUMMARY AND RECOMMENDATION FOR FUTURE WORK ............................................................................................. 174 Chapter 10. Research summary and recommendation for future work ... 175 10.1 Introduction ....................................................................................... 175 10.2 Research summary ............................................................................. 175 10.3 Recommendation for future work....................................................... 179 10.3.1 Future work for astaxanthin production by C. zofingiensis ........... 179 10.3.2 Future work for biodiesel production by C. zofingiensis ............... 180 REFERENCES................................................................................................. 183 x LIST OF ABBRECIATIONS ACCase acetyl-CoA carboxylase ACP acyl carrier protein BC biotin carboxylase BKT carotenoid ketolase CHYb carotenoid hydroxylase CN cetane number CP cloud point CPTA 2-(4-chloro-phenylthio)-triethylamine DAG diacylglycerol DMAPP dimethylallyl diphosphate DPA diphenylamine DTT dithiothreitol DUS the degree of fatty acid unsaturation EB ethidium bromide EMS ethyl methanesulphonate FAME Fatty acid methyl ester FP flash point FPP farnesyl diphosphate GC gas chromatography GGPP geranylgeranyl pyrophosphate GGPS geranylgeranyl pyrophosphate synthase GPP geranyl diphosphate GL glycolipid HPLC high performance liquid chromatography IPP isopentenyl diphosphate KAS 3-ketoayl ACP synthase KV kinematic viscosity xi LCY lycopene cyclase MAG monoacylglycerol MNNG N-methyl-N′-nitro-N-nitrosoguanidine MUFA monounsaturated fatty acid NL neutral lipid NPQ non-photochemical quenching NXS neoxanthin synthase PDS phytoene desaturase PL phospholipid PP pour point PSY phytoene synthase PUFA polyunsaturated fatty acid RACE rapid amplification of cDNA ends RNS reactive nitrogen species ROS reactive oxygen species RT-PCR reverse transcription polymerase chain reaction SAD stearoyl ACP desaturase SE steroid ester SFA saturated fatty acid TAG triacylglycerol TC total carotenoids TFA total fatty acids TLC thin layer chromatography UV ultraviolet WT wild type ZEP zeaxanthin epoxidase xii PART I LITERATURE REVIEW AND RESEARCH AIM 1 Chapter 1. Literature review and research aim Chapter 1 Literature review and research aim 1.1 Introduction Algae are a large and diverse group of unicellular or multicellular organisms, ranging from the microscopic cyanobacteria (commonly referred to as blue-green algae), which are closely related to Gram-negative bacteria, to the giant kelps with the height of over 10 m (Graham et al., 2009). Like plants, algae are photoautotrophic, yet some are also able to grow heterotrophically at the expense of organic carbon sources such as sugars; in addition, some algae can be mixotrophic, with the ability of utilizing both organic carbon substrate and light and CO2 for growth (Radmer, 1996). Algae are dominant in water, common on land and can also be found in unusual environments, such as on snow and in desert soils (Lee, 2008). Microalgae represent one of the most promising sources for numerous products including carotenoids, polyunsaturated fatty acids, proteins, polysaccharides, vitamins, and other biologically active compounds, and thus are used in a wide variety of technological applications for the development of feed and food products (Pulz & Gross, 2004; Gouveia et al., 2009). They are also found to be useful in bioremediation applications for environmental clean-up (Suresh & Ravishankar, 2004; Munoz & Guieysse, 2006). Recently, microalgae have been considered with a great potential as a sustainable feedstock superior to oil crops for biodiesel production (Chisti, 2007). This chapter reviews the background of astaxanthin, approaches through genetic engineering for enhanced astaxanthin production, current status of biodiesel and the production potential of biodiesel using microalgae as feedstocks. 2 Chapter 1. Literature review and research aim 1.2 Astaxanthin Astaxanthin is a red ketocarotenoid that belongs to the family of the xanthophylls. It can be found in some microalgae, bacteria, yeasts, and many marine animals (Lorenz & Cysewski, 2000; Zhang et al., 2009). Because of its strong pigmentation function, powerful antioxidative activity and beneficial effects on human health, astaxanthin has important applications in feed, food, nutraceutical, and pharmaceutical industries (Guerin et al., 2003; Fraser & Bramley, 2004). 1.2.1 Biochemical properties of astaxanthin Carotenoids are a group of structurally diverse terpenoid pigments with the isoprene as basic units. The structure of carotenoids is derived from lycopene and the majority is the 40-carbon chain conjugated by double bonds (Figure 1.1). Carotenoids are split into two classes, carotenes which are purely hydrocarbons (e.g., lycopene and β-carotene) and xanthophylls which are oxygenated derivatives of carotenes (e.g., zeaxanthin, canthaxanthin and astaxanthin) (Jin et al., 2003). 3 Chapter 1. Literature review and research aim Lycopene β-carotene OH Zeaxanthin OH O O Canthaxanthin O OH OH O Astaxanthin Figure 1.1 Chemical structure of some carotenoids (Sandmann, 2001). Asatxanthin is a hydrophobic carotenoid formed via the hydroxylation and oxidation of β-carotene, with a chemical formula of C40H52O4 and a molecular weight of 596.86 (Britton et al, 2004). It exists in geometric cis and trans isomers; the latter is thermodynamically more stable than the former and found predominantly in nature (Britton, 1995). As for the trans astaxanthin, there are three forms of stereoisomers: two enantiomers (3R, 3′R and 3S, 3′S) and a meso form (3R, 3′S) (Higuera-Ciapara et al., 2006). The isomers have the same molecular and structural formula with the only difference in position of the 4 Chapter 1. Literature review and research aim rotating functional group (Figure 1.2). Among these isomers, the 3S, 3′S is the most abundant in nature, predominantly synthesized by green microalgae (Lorenz & Cysewski, 2000). In contrast, synthetic astaxanthin is a racemic mixture of these three isomers (Turujman et al., 1997). Figure 1.2 Astaxanthin configurational isomers and its geometric cis isomer (Higuera-Ciapara et al., 2006). 5 Chapter 1. Literature review and research aim Astaxanthin exists in free form or esterified in its one or both hydroxyl groups with various fatty acids such as palmitic, stearic, and oleic. Synthetic astaxanthin is in free form while the natural one found in algae is preferred in esterified form (Miao et al., 2006; Peng et al., 2008; Holtin et al., 2009). In addition, astaxanthin found in marine animals may interact with proteins or lipoproteins to form carotenoproteins or carotenolipoproteins (Ando & Tanaka, 1996; Britton et al., 1997). 1.2.2 Applications of astaxanthin 1.2.2.1 Astaxanthin as a coloring agent With strong coloring ability, astaxanthin serves as the feed supplement and food additive for aquaculture. The use of astaxanthin in aquaculture species, for example salmon and lobster, has been extensively studied and well documented in the past years (Torrissen, 1986; Laird et al., 2001; Wade et al., 2005; Bjerkeng et al., 2007; Williams, 2007; Niamnuy et al., 2008; Choubert & Baccaunaud, 2010). Human and animals cannot synthesize carotenoids de novo, instead, they have to obtain carotenoids through their food chain or feeds. The dietary carotenoids give such organisms as salmonids and crustacean the reddish-orange color characteristic that is regarded by consumers as one of the key quality attributes (Laird et al., 2001). Astaxanthin is the major carotenoid found in marine animals, for example in crab the red pigment accounts for more than 80% of total carotenoids (Shahidi & Synowiecki, 1991). Because of the higher color intensity and better absorption by the digestive tract of salmonids, astaxanthin is preferred over canthaxanthin in aquatic farming (Torrisen, 1986; Storebakken & No, 1992). In addition to pigmentation, astaxanthin has been shown to benefit the growth and survival of larval fish and shrimp (Storebakken & Goswami 1996; Niu et al., 2009). Astaxanthin is also used in the tropical marine 6 Chapter 1. Literature review and research aim ornamental industry and poultry, aiming to pigment ornamental fish or egg yolk (Elwinger et al., 1997; Ako & Tamaru, 1999; Fredriksson et al., 2006). 1.2.2.2 Astaxanthin as an antioxidant During normal metabolic processes for energy production, oxygen is reduced, producing several oxygen-derived free radicals such as hydroxyls and peroxides, as well as reactive oxygen species (ROS) which are considered playing important roles in hormone biosynthesis, cell signaling and aging, and microbial killing (Rada & Leto, 2008). ROS levels can increase dramatically when exposing to environmental stress, for example UV or heat. Without efficient removal, the overproducing ROS become a problem, resulting in oxidative stress that causes DNA damage, protein and lipid oxidation, and inactivation of specific enzymes (Stadtman, 1992; Papas, 1999). Such oxidative damage has been linked to various diseases, for example, retinopathy, carcinogenesis, arteriosclerosis, and age-related macular degeneration (Papas, 1999; Muller et al., 2007). In addition to the enzymatic antioxidants generated by bodies (e.g., super oxidate dismutase, catalase, and peroxidase), dietary antioxidants such as carotenoids can serve as potent free-radical scavengers to cope with the oxidative stress and benefit human health. The powerful antioxidant activity of astaxanthin has been demonstrated in numerous studies in the past years (Kurashige, 1990; Palozza & Krinsky, 1992; Lawlor & O'Brien, 1995; Shimidzu et al., 1996; Naguib, 2000; Kupcinskas et al., 2008; Liu et al., 2009a). It was reported that astaxanthin has stronger anti-oxidative activity than other carotenoids and vitamin E (Kurashige, 1990; Palozza & Krinsky, 1992; Naguib, 2000). The strong anti-oxidative power of astaxanthin is indicated by the oxygen radical absorbance capacity (ORAC) value as shown in Figure 1.3. It is the powerful anti-oxidative properties that make astaxanthin play important roles in following human health conditions: 7 Chapter 1. Literature review and research aim (1) reducing the DNA damage (Santocono et al., 2006, 2007; Tripathi & Jena, 2009); (2) protecting eyes and skin from UV-light mediated photo-oxidation (Lyons & O'Brien, 2002); (3) protecting membranes of cell and mitochondria from oxidative damage (Barros et al., 2002; Goto et al., 2001; Liu et al., 2009a; Wolf et al., 2010); (4) inhibiting lipid peroxidation that may cause plaque formation in the circulatory system (Goto et al., 2001; McNulty et al., 2007); (5) attenuating inflammation by quenching ROS (Bennedsen et al., 2000; Lockwood et al., 2006; Pashkow et al., 2008); (6) crossing the blood-brain barrier in mammals and alleviates oxidative stress, and may help maintain neuroligic health (Hussein et al., 2005; Liu et al., 2008); (7) benefiting liver function by pretecting liver cells against oxidative damage (Gradelet et al., 1998; Curek et al., 2010); (8) preventing the initiation of tumorigenesis in the mouth, oral cavity, prostate, large bowel, bladder and breast (Tanka et al., 1994, 1995; Lockwood et al., 2006); (9) boosting immune system by enhancing the production of antibody and increase the total number of T-cells (Jyonouchi et al., 1993, 1994, 1995); (10) benefiting heart health by modifying blood levels of LDL and HDL cholesterol (Miki et al., 1998; Yoshida et al., 2010). 8 Chapter 1. Literature review and research aim Figure 1.3 ORAC values of carotenoids and vitamin E. ORAC is a method for measuring antioxidant ability of foods and other chemicals using Trolox as the standard. A higher ORAC value represents a higher antioxidant ability (Ip, 2005). 1.2.3 Potential sources of astaxanthin Being widely used in aquatic farming as a feed additive, astaxanthin market in United States is estimated to be US$200 millions per year at a price of around US$2500/kg (Lorenz & Cyswski, 2000). Commercial astaxanthin can be produced synthetically or extracted from crustacean byproducts and some microorganisms. 9 Chapter 1. Literature review and research aim 1.2.3.1 Synthetic astaxanthin Nowadays, commercial astaxanthin for aquaculture is mainly produced synthetically from petrochemical sources, whereas natural astaxanthin contributes only to a minor portion of the market (Guerin et al., 2003). Synthetic astaxanthin contains a mixture of isomers (3S, 3′S), (3S, 3′R), and (3R, 3′R) at the ratio of 1:2:1 (Alga Technologies, 2004). DSM and BASF are the world’s leading suppliers of synthetic astaxanthin. Although the astaxanthin market is occupied by synthetic products, the growing demand of customers for natural foods has urged the production of astaxanthin from natural sources such as crustacean by-products, yeast, microalgae and transgenic plants. 1.2.3.2 Astaxanthin from crustacean by-products Crustacean by-products such as heads, shells and tails generated from food processing or conditioning consist mainly of chitin, proteins, fatty acids and carotenoids (Heu et al., 2003). The carotenoid composition and content in crustacean by-products have been well studied (Mandeville et al., 1991; Shahidi & Synowiecki, 1991; Olsen & Jacobsen, 1995; Sachindra & Mahendrakar, 2005; Sachindra et al., 2007; Babu et al., 2008; Handayani et al., 2008), indicating the potential of these by-products as natural astaxanthin source. However, these by-products generally contains only a small amount of astaxanthin (Table 1.1) but high contents of ash and chitin that significantly decrease fish’s digestibility (Higuera-Ciapara et al., 2006), making the use of crustacean by-products in fish feeding less feasible and economical. Astaxanthin found in crustacean by-products is mainly in esterified form (Table 1.1). 10 Chapter 1. Literature review and research aim Table 1.1 Carotenoid contents in various sources of crustacean by-products. Adapted from Higuera-Ciapara et al. (2006) Total astaxanthin (μg/100 g) Source Shrimp (P. borealis) Shrimp (P. borealis) Crawfish (P. clarkii) Backs snow crab (Ch. opilio) a Astaxanthin (%) Other carotenoids Free Monoester Diester 14.77 4.0 19.7 74.3 zeaxanthin 4.97a 8 22.5 69.5 Not detected 15.3 40.3 11.96 21.2 49.4 astacene 5.1 56.6 Lutein, zeaxanthin, astacene mg/100 g wet basis 1.2.3.3 Astaxanthin from yeast The red yeast Xanthophyllomyces dendrorhous (previously referred as Phaffia rhodozyma) as the astaxanthin producer has been intensively studied in the past years (Cruz & Parajo, 1998; Parajo et al., 1998; An et al., 2001; Ramirez et al., 2001; Visser et al., 2003; Zheng et al., 2006; Liu & Wu, 2007; Lee et al., 2008). X. dendrorhous can grow fast and achieve high cell biomass through utilizing a variety of carbon sources such as glucose, xylose and even molasses. However, the cellular astaxanthin content is relatively low, varying from 0.14 to 0.79 mg g-1 dry weight depending on different strains of X. dendrorhous (Ip, 2005). Genetic modification strategies have been employed to enhance the astaxanthin content in X. dendrorhous cells (An et al., 1989; An et al., 1996; Ramirez et al., 2000; Ukibe et al., 2008). Ukibe et al. (2008) addressed that the isolated mutants of X. dendrorhous could produce 1.5-3.8 fold more astaxanthin than the wild type cells. Currently X. dendrorhous is commercial in a fine powder form as a natural source of astaxanthin for fish feeding. The thick cell walls of yeast, however, hinder the assimilation of astaxanthin by fish and thus cell wall 11 Chapter 1. Literature review and research aim disruption is needed (Storebakken et al., 2004). The X. dendrorhous derived astaxanthin is exceptionally in the isomer form of 3R, 3′R (Johnson & An, 1991). 1.2.3.4 Astaxanthin from microalgae Microalgae represent the most potential sources of natural astaxanthin and have attracted thorough investigations in terms of strain screening, culture medium optimization, stress induction, cultivation strategy modification and genetic improvement for astaxanthin accumulation and production during the past decades (Boussiba et al., 1992; Olaizola, 2000; Orosa et al., 2000; Hata et al., 2001; Orosa et al., 2001; Ip and Chen, 2005; Steinbrenner & Sandmann, 2006; Hu et al., 2008; Sandesh Kamath et al., 2008; Sun et al., 2008; Zhang et al., 2009). Heamatococcus pluvialis has been considered as the most promising microalga for commercial astaxanthin production, in that it is able to accumulate astaxanthin up to 4% of its dry biomass, the highest content in nature (Boussiba, 2000). Heamatococcus algal meal has been approved as a color additive in salmonid feeds and as a dietary-supplement ingredient for human consumption in Japan, USA and some other countries. In the large-scale, enclosed photobioreactor or outdoor system, a two-step process is employed for the production of astaxanthin-rich Heamatococcus cells (Figure 1.4): cell biomass accumulation and astaxanthin induction. First, vegetable cells accumulate and achieve a sufficient density under optimal growth conditions; the cell abundant culture then is subjected to stress conditions (e.g., deprivation of nitrate, high light intensity, and salt stress) for astaxanthin induction (Fabregas et al., 2001; Zhang et al., 2009). 12 Chapter 1. Literature review and research aim Figure 1.4 Typical flow sheet for the commercial production of Haematococcus derived natural astaxanthin (Lorenz & Cysewski, 2000). Although H. pluvialis can accumulate a high content of astaxanthin, it grows relatively slow with a low cell biomass yield and is susceptible to contamination and adverse environment (Lorenz & Cysewski, 2000; Olaizola, 2000; Hata et al., 2001; Ip, 2005). In addition, extremely high light illumination is required for astaxanthin induction and accumulation in this alga and thus hindering its commercial application (Fabregas et al., 2001; Imamoglu et al., 2009). Recently, the green alga Chlorella zofingiensis has been regarded as a potential alternative host for mass production of astaxanthin due to its fast growth, low sensitivity to contamination and unfavorable environments, and astaxanthin 13 Chapter 1. Literature review and research aim accumulation under heterotrophic conditions with glucose as the sole carbon and energy source (Rise et al., 1994; Bar et al., 1995; Orosa et al., 2001; Del Campo et al., 2004; Ip & Chen, 2005; Sun et al., 2008). In addition, another green microalga Chlorococcum sp. was reported to produce astaxanthin in photoautotrophic cultures as well as in heterotrophic and mixotrophic cultures on glucose (Ma, 2001). 1.2.3.5 Astaxanthin from transgenic plants With the exception of Adonis which produces astaxanthin in its flower petals (Seybold & Goodwin, 1959), plants are unable to synthesize astaxanthin because they are devoid of the β-carotene ketolase that induce keto-moieties to the 4, 4’ position of β-ionone rings of β-carotene and zeaxanthin. However, the production of astaxanthin can be achived through the functional expression of a heterologous β-carotene ketolase gene in plants. There are numerous studies addressing the accumulation of astaxanthin in transgenic plants including tobacco (Mann et al., 2000; Ralley et al., 2004; Gerjets et al., 2007; Hasunuma et al., 2008), Arabidopsis (Zhong et al., 2008), potato (Gerjets & Sandmann, 2006; Morris et al., 2006), carrot (Jayaraj et al., 2008) and even tomato (unpublished data from our laboratory). Although astaxanthin is produced in low amount in transgenic plants, the accumulated astaxanthin adds nutritional value to the edible parts of crop plants for human health and may reach commercial use in the near future. 1.2.3.6 Natural astaxanthin versus synthetic astaxanthin The difference between natural and synthetic astaxanthin lies in their stereochemical orientation. Synthetic astaxanthin is a mixture of three isomeric forms with 50% being 3R, 3′S, while natural astaxanthin from microalgae is in the 14 Chapter 1. Literature review and research aim form of 3S, 3′S. The 3S, 3′S form of astaxanthin is reported to give a stronger pigmentation to rainbow trout than other forms and is thus preferred as feed additives for fish farming (Osterlie et al., 1999). Haematococcus astaxanthin is considered to play important roles in human health and nutrition, while for other isomers no significant biological effect has been established (Guerin et al., 2003). Moreover, unlike synthetic astaxanthin that is present in free form, natural astaxanthin usually exists mono-esterified or di-esterified with fatty acids (Miao et al., 2006; Peng et al., 2008; Holtin et al., 2009). The esterified astaxanthin is inherently more stable than the free one, thus giving a greatly longer shelf life without being oxidized. As consumers become more and more aware of the putative benefits of natural astaxanthin, and as the commercial production is optimized with lowered costs, the natural astaxanthin will beat petroleum derived synthetic astaxanthin and finally dominate the market. 1.2.4 Genetic modification for enhanced astaxanthin accumulation 1.2.4.1 Mutagenesis Mutagenesis is a process by which the genetic information of an organism is changed in a stable manner, either in nature or experimentally by the use of chemicals or radiation. Ethyl methanesulphonate (EMS) and N-methyl-N′-nitro-N-nitrosoguanidine (MNNG) are the most preferred chemical mutagens. Ultraviolet (UV) light is also frequently used. Mutagenesis has been widely used to modify X. dendrorhous for improved astaxanthin production (An et al., 1989; Lewis et al., 1990; An et al., 1996; Bon et al., 1997; Ramirez et al., 2000; Ukibe et al., 2008). X. dendrorhous mutants could produce 1-4 fold more astaxanthin as compared with the parent ones (Lewis et al., 1990; Bon et al., 1997; Ukibe et al., 2008). Mutagenesis has also been employed to enhance the biosynthesis of carotenoids including astaxanthin in various algal species, 15 Chapter 1. Literature review and research aim especially in H. pluvialis (Tjahjono et al., 1994; Zhang & Lee, 1997; Chen et al., 2003; Ishikawa et al., 2004; Hu et al., 2008; Sandesh Kamath et al., 2008). Commonly, after treatment of mutagens, specific inhibitors (mainly herbicide, e.g., fluridone, norflurazon, nicotine and diphenylamine) to carotenogenic enzymes are used to select mutants with enhanced biosynthesis of carotenoids. Hu et al. (2008a) reported an astaxanthin-overproducing mutant of H. pluvialis that could accumulate astaxanthin about two times of that by the wild type (WT). 1.2.4.2 Genetic engineering of the carotenoid biosynthetic pathway Within the past few years, genetic engineering of carotenoid metabolic pathway has been widely employed in bacteria, algae and plants to increase the amount of pre-existing carotenoids (Shewmaker et al., 1999; Steinbrenner & Sandmann, 2006), to alter the carotenoid contents (Albrecht et al, 1999; Romer et al., 2000; Gerjets et al., 2007), and to biosynthesize new products (Harker & Hirschberg, 1997; Mann et al., 2000; Ye et al., 2000; Ralley et al., 2004; Morris et al., 2006; Gerjets & Sandmann, 2006; Hasunuma et al., 2008; Jayaraj et al., 2008; Zhong et al., 2008). Several prerequisites are required for the engineering of the carotenoid metabolic pathway: (1) a clearly elucidated map for carotenoid biosynthesis; (2) a collection of cloned genes encoding the enzymes required in the carotenoid biosynthetic pathway; (3) knowledge about the cellular localization of carotenoids and the enzymes involved in the carotenoid biosynthesis. 1.2.4.2.1 A clearly elucidated map for carotenoid biosynthesis Up till now, major advances have been made in the elucidation of carotenoid biosynthetic pathway in bacteria, algae and higher plants. It is widely accepted that in these organisms the biosynthesis of primary carotenoids follows a similar pathway, which generally involves three types of reactions: condensation of two molecules of geranylgeranyl pyrophosphate (GGPP) resulting in the first 16 Chapter 1. Literature review and research aim C40 carotene phytoene, four step-wise desaturation reactions converting colorless phytoene to pink-colored lycopene, and cyclization of this pigment to introduce β-ionone or ε-ionone groups at both ends, leading to the formation of β-carotene and α-carotene respectively (Sandmann, 2002). A map for the biosynthesis of β-carotene and α-carotene is outlined in Figure 1.5. Xanthaphylls, deriving from oxidation of carotenes (β-carotene and α-carotene), are different in diverse types of organisms, for example higher plants generally lack loroxanthin, astanxanthin and canthaxanthin which are produced by certain green algae under specific environmental stimuli (Jin et al., 2003). Basically several hydroxylation, oxygenation and epoxidation reactions are involved in the synthesis of xanthophylls, which is showed in Figure 1.6. Hydroxylation of the C-3 and C-3′ positions of β-carotene and α-carotene result in the formation of zeaxanthin and lutein via β-cryptoxanthin and α-cryptoxanthin, respectively. The subsequent epoxidation of zeaxanthin leads to the production of violaxanthin which is further converted to neoxanthin. While in some algae, additional xanthaphyll biosynthetic pathway is present, for example, oxygenation and hydroxylation of β-carotene brings on the synthesis of astaxanthin, a commercially high-value ketocarotenoid. 1.2.4.2.2 A collection of cloned genes encoding the enzymes required in the carotenoid biosynthetic pathway In the past few years, genes encoding most of the enzymes involved in the carotenoid biosynthesis from different prokaryotic and eukaryotic organisms have been cloned and identified (Table 1.2; reviews see Armstrong, 1997; Cunningham & Gantt, 1998; Jin et al, 2003). The availability of these genes has greatly facilitated the genetic manipulation of carotenoid biosynthesis for special purposes, such as the production of a new pigment astaxanthin in tobacco flowers and leaves (Mann et al, 2000; Ralley et al., 2004). Since the genes from bacteria share low level identity at nucleotide sequences with those from plants thus eliminating the co-suppression commonly present in transgenic plants, the 17 Chapter 1. Literature review and research aim CH2OPP CH2OPP DMAPP IPP + IPP CH2OPP GPP + IPP CH2OPP FPP + IPP CH2OPP GGPP + GGPP Phytoene Phytofluene ζ-carotene Neurosporene Lycopene α-carotene β-carotene Figure 1.5 Carotenoid biosynthetic pathway to form β-carotene and α-carotene (Sandmann, 2002). Abbreviations: DMAPP, dimethylallyl diphosphate; IPP, isopentenyl diphosphate; GPP, geranyl diphosphate; FPP, farnesyl diphosphate; GGPP, geranylgeranyl diphosphate. 18 Chapter 1. Literature review and research aim Lycopene α-carotene β-carotene Echinenone Adonirubin Cryptoxanthin Lutein Zeaxanthin Loroxanthin Antheraxanthin Canthaxanthin Adonixanthin Astaxanthin Violaxanthin Neoxanthin Figure 1.6 Schematic diagram of pathway of xanthophyll biosynthesis. In the box is the astaxanthin biosynthetic pathway which is present in certain algae. Two possible ways are indicated. Adapted from Jin et al. (2003). bacteria-derived genes have been successfully employed to transform plants for enhanced production of carotenoids. For example, overexpression of the phytoene synthase gene from bacterium Erwinia uredovora (crtB) leaded to 2-4-fold higher total carotenoids in fruits of transgenic tomato plants (Fraser et al., 2002); constitutive expression of crtI (phytoene desaturase gene from E. uredovora) in rice transformants resulted in the accumulation of α-carotene, β-carotene, lutein and zeaxanthin in endosperms that do not normally produce carotenoid pigments (Ye et al., 2000); simultaneous expression of crtZ and crtW from Agrobacterium aurantiacum as polyprotein made tobacco accumulate new carotenoids including astaxanthin, canthaxanthin and 4-ketozeaxanthin (Ralley et al., 2004). The cyanobacterium derived crtO gene was also expressed in potato, resulting in the 19 Chapter 1. Literature review and research aim accumulation of echinenone, 3'-hydroxyechinenone, and 4-ketozeaxanthin in leaves, as well as astaxanthin in the tuber (Gerjets & Sandmann, 2006). In addition, astaxanthin-producing transgenic potato and carrot plants were successfully obtained through the expression of a β-carotene oxygenase gene from H. pluvialis, making these economical crops more nutritionally attractive (Morris et al., 2006; Gerjets et al., 2008). All the mentioned transgenic plants are generated through Agrobacterium-mediated gene transfer method, yielding low amounts of astaxanthin due to the presence of large quantities of astaxanthin intermediates. The employment of plastid transformation strategy overcame this problem and made transgenic tobacco plants accumulate high levels of astaxanthin (more than 70% of total carotenoids) in leaves (Hasunuma et al., 2008). In addition to higher plants, the green alga H. pluvialis has also been successfully genetically modified with accelerated astaxanthin production (Steinbrenner & Sandmann, 2006). 20 Chapter 1. Literature review and research aim Table 1.2 Genes involved in the carotenoid biosynthesis Gene Enzymatic function Species crtE/GGPPS GGPP synthase Erwinia uredovora (Misawa et al., 1990); Pepper (Kuntz et al., 1992); Arabidopsis (Lange & Ghassemian, 2003) crtB/PSY Phytoene synthase E. ruedovora (Misawa et al., 1990); Arabidopsis (Lange & Ghassemian, 2003); Tomato (Fraser et al., 2000); H. pluvialis (Steinbrenner & Linden, 2001) crtI/crtP/PDS Phytoene desaturase E. ruedovora (Misawa et al., 1990); Synechococcus (Chamovitz et al., 1991); Chlamydomonas reinhardtii (McCarthy et al., 2004), H. pluvialis (Harker & Hirchberg, 1997); C. zofingiensis (Huang et al., 2008); Dunaliella salina (Zhu et al., 2005); Arabidopsis (Lange & Ghassemian, 2003); Tomato (Pecker et al., 1992) crtY/crtL/LCY Lycopene cyclase E. ruedovora (Misawa et al., 1990); Synechococcus (Cunningham et al., 1994); C. reinhardtii (Lohr et al., 2005); H. pluvialis (Steinbrenner & Linden, 2003); D. salina (Ramos et al., 2008); Arabidopsis (Cunningham et al., 1996; Cunningham & Gantt, 2001); Tomato (Rogen et al., 1999 & 2000) crtZ/CHYb crtW/crtO/BKT β-carotene E. ruedovora (Misawa et al., 1990); C. reinhardtii (Lohr et al., 2005); H. pluvialis (Linden, 1999); C. zofingiensis hydroxylase (Li et al., 2008); Arabidopsis (Sun et al., 1996); Tomato (Hirchberg, 1998) β-carotene oxygenase A. aurantiacum (Misawa et al., 1995); Synechocystis (Fernandez-Gonzalez et al., 1997); C. reinhardtii (unpublished data); H. pluvialis (Kajiwara et al., 1995; Huang et al., 2006a); C. zofingiensis (Huang et al., 2006b); ZEP Zeaxanthin epoxidase Arabidopsis (Lange & Ghassemian, 2003); Pepper (Bouvier et al., 1996) NXS Neoxanthin synthase Potato (Al-Babili et al., 2000); Tomato (Bouvier et al., 2000) 21 Chapter 1. Literature review and research aim 1.2.4.2.3 Cellular localization of carotenoids and the enzymes involved in the carotenoid biosynthesis Carotenoids, relatively hydrophobic molecules, are typically associated with membranes and/or non-covalently bound to specific proteins (Armstrong, 1997). Considerable progress has been made in the cellular localization of carotenoids and the enzymes that are required for the synthesis of these carotenoids in the recent years. In higher plants, carotenoids are localized in the plastid where the carotenoid biosynthetic enzymes are present as membrane associated or bound proteins. Phytoene synthase, responsible for the formation of the first carotenoid phytoene from GGPP through a two-step reaction, was purified from pepper chromoplast stroma in soluble form (Dogbo et al., 1988). The solubilization of this enzyme, however, required treatment with high ionic strength buffer or mild non-ionic detergent (Fraser et al., 2000), suggesting that phytoene synthase acitivity is membrane-associated. As reported by Al-Babili et al. (1996), both soluble and membrane-bound phytoene desaturase was detected by western blot analysis in the isolated chromoplasts: the soluble one in stroma showed no enzymatical activity and got activated once bound to membrane. This is further supported by in vitro protein import assays in pea chloroplasts (Bonk et al., 1997). In this research, the suborganellar localization of three other carotenogenic enzymes was also investigated. Similar to phytoene desaturase, after imported into chloroplasts, lycopene cyclase remained soluble in the stroma, forming a high-molecular-mass complex with chloroplast 60-kDa chaperonin (Cpn60); while phytoene synthase transiently formed a complex with Cpn60 and then associated rapidly to thylakoid membranes upon import. As expected, the geranylgeranyl diphosphate synthase stayed in soluble and free form in the stroma. The in vitro import assay of carotenogenic enzymes from citrus was also carried out in a very recent report which demonstrated the subcellular localization and transit peptide cleavage of these proteins (Inoue et al., 2006). Consistent with the 22 Chapter 1. Literature review and research aim results stated above, phytoene synthase was found to be peripherally associated with the membrane and phytoene desaturase mainly stayed in the stroma. Lycopene cyclase, however, was targeted both to the soluble and to the membrane compartments, which slightly differed from the previous report of Bonk et al. (1997). In addition, carotenoid β-ring hydroxylase was exclusively inserted into the chloroplast internal membranes, the first demonstration of plastid cellular localization of this enzyme in plants (Inoue et al., 2006). Similar to plants, in algae all primary carotenoids and some xanthaphylls such as lutein and zeaxanthin are synthesized within plastids. Additional carotenoids, e.g. canthaxanthin and astaxanthin which are generally not present in plants, however, are found to accumulate in lipid vesicles outside the plastid in H. pluvialis (Boussiba, 2000). The cellular compartmentaton of H. pluvialis phytoene desaturase was investigated through using immunogold labeling of ultra sections and western blot analysis of cell fractions which revealed that this active enzyme was localized exclusively in the chloroplast, or more specifically speaking in close contact to thylakoids (Grunewald et al., 2000), ruling out the possible biosynthesis of lycopene, the direct β-carotene precursor, outside chloroplast. β-carotene oxygenase, the enzyme catalyzing the introduction of keto groups at position C-4 of the β-ionone ring of β-carotene and zeaxanthin, localized both in the chloroplast and cytoplasmic membrane-derived lipid vesicles but only functions in the latter compartment (Grunewald et al., 2001). Based on the information, there might be a transport process of intermediate carotenoids over compartment borders from chloroplast as the site of synthesis to the lipid vesicles located in the cytoplasm as the site of accumulation. To determine what carotenoids may serve as the intermediates, further research was carried out by Grunewald & Hagen (2001). Treated with diphenylamine (DPA, a specific inhibitor to β-carotene oxgenation), the majority of β-carotene was found to accumulate in lipid vesicles; while few lycopene was observed when treated with 2-(4-chlorophenylthio)-triethylamine (CPTA, specifically inhibiting lycopene cyclization), suggesting that β-carotene is the intermediate exported from the 23 Chapter 1. Literature review and research aim chloroplast during accumulation of secondary carotenoids in H. pluvialis. It was hypothesized that the nuclear-encoded β-carotene oxgenase could be transported first into the chloroplast and might then be exported out together with the substrate β-carotene into cytoplasm where it was sequestered by cytoplasmic membrane-derived lipid vesicles (Grunewald et al., 2001). Since the β-carotene hydroxylase owns a dual function in H. pluvialis being responsible for the formation of zeaxanthin from β-carotene and of astaxanthin from canthaxanthin, it may locate and function in both the chloroplasts and the lipid vesicles. Generally, enzymes should locate specific cellular compartments where substrates and/or cofactors are present to perform their function. Thus the information about cellular compartmentation described above is of great importance to genetic manipulation of carotenoid biosynthesis. Since carotenoids are produced and accumulated in plastids, the heterogenous genes which are employed to genetically engineer carotenoid biosynthesis in certain organism should be fused to a short specific protein sequence that ensures the plastid-targeting of enzymes encoded by these genes. Overexpression of a bacterial phytoene synthase (crtB) gene resulted in 50-fold higher carotenoid accumulation in canola mature seed (Shewmaker et al., 1999). crtI, another bacteria gene encoding a enzyme responsible for a four-step desaturation reaction, has been widely used in plants to elevate carotenoid level or produce new pigments in carotenoid-free tissues (Romer et al., 2000; Ye et al., 2000). In these genetic manipulations or others (Gerjets & Sandmann, 2006; Morris et al., 2006), the pea ribulose bisphosphate carboxylase small subunit transit sequence was used to target the proteins of interest into plastids. Tomato phytoene synthase-1 transit sequence was also employed for the charomoplast targeting of CRTB protein to alter the carotenoid content (Fraser et al., 2002). What’s more, the crtO gene from green algae H. pluvialis was transferred to tobacco and the CRTO polypeptide was targeted by the transit peptide of PDS from tomato into chromoplasts where this enzyme catalyzes the oxygenation reaction, leading to the accumulation of astaxanthin and other ketocarotenoids in nectary tissues and thus the color of the 24 Chapter 1. Literature review and research aim nectary changes from yellow to red (Mann et al., 2000). 1.3 Biodiesel 1.3.1 Introduction Fossil-based fuels including oil, coal and gas play a pivotal role in modern world energy market. These fossil fuels, according to world energy outlook 2007, will remain the major sources of energy and are expected to meet about 84% of energy demand in 2030. However, fossil fuels are non-renewable and will be finally diminished. It has been recently estimated that the global oil, coat and gas lasts only around for 35, 100 and 37 years respectively, based on a modified Klass model (Shafiee & Topal, 2009). In order to sustain a stable energy supply in the future, it is necessary to develop other sources of energy, e.g., renewable energy. Renewable energy is derived from natural processes that are replenished constantly, including hydropower, wind power, solar energy, geothermal energy, biodiesel, etc. An estimated $120 billion was invested in renewable energy worldwide in 2008, around 2 times of the 2006 investment (Figure 1.7). 25 Chapter 1. Literature review and research aim Figure 1.7 Global investment in renewable energy, 2004-2008. Source: REN21, 2009. It is well known that transport is almost totally dependent on petroleum-based fuels, which will be depleted within no more than 40 years. An alternative fuel to petrodiesel must be technically feasible, easily available, economically competitive, and environmentally acceptable (Demirbas, 2008). Biodiesel is such a candidate fuel for powering the transport vehicles. Biodiesel refers to a biomass-based diesel fuel consisting of long-chain alkyl (methyl, propyl or ethyl) esters. In addition to being comparable to petrodiesel in most technical aspects, biodiesel has several following distinct advantages over petrodiesel (Knothe, 2005): (1) derivation from renewable domestic resources, thus reducing dependence on and preserving petroleum; (2) biodegradability; (3) reduction of most exhaust emissions (except nitrogen oxides, NOx). 26 Chapter 1. Literature review and research aim (4) higher flash point, leading to safer handling and storage; (5) excellent lubricity. Like petrodiesel, biodiesel operates in compression ignition engines. Biodiesel is miscible with petrodiesel in all ratios. Currently, the blends of biodiesel and petrodiesel instead of net biodiesel have been widely used in many countries and no engine modification is required (Singhania et al., 2008). These blends of biodiesel with petrodiesel are usually denoted by acronyms, for example B20 which indicates a blend of 20% biodiesel with petrodiesel (Knothe, 2005). The global markets for biodiesel are entering a period of rapid and transitional growth. In the year 2007, there were only 20 oil producing nations supplying the needs of over 200 nations; by the year 2010, more than 200 nations will become biodiesel producing nations and suppliers (Thurmond, 2008). Global biodiesel production has massively increased to 10.8 million tons per year over the last eight years (Figure 1.8). Much of the growth is happening in just three countries: the United States, Brazil and Germany, which together account for over half of biodiesel (Checkbiotech, 2009). The International Energy Agency’s report suggests that world production of biodiesel could top 20 million tons per year by 2012 if the recent trends continue. 27 Chapter 1. Literature review and research aim Figure 1.8 Global biodiesel production, 2001-2008. Adapted from Thurmond (2008). 1.3.2 Biodiesel production through transesterification Vegetable oils have long been recognized as fuels for diesel engines (Shay, 1993). However, vegetable oils are too viscous for most currently used diesel engines. The widely employed method in industry to reduce oil viscosity is called transesterification, resulting in biodiesel production (Demirbas, 2005). Transesterification is a chemical conversion process involving reacting triglycerides of vegetable oils or animal fats catalytically with a short-chain alcohol (typically methanol or ethanol) to form fatty acid esters and glycerol (Figure 1.9). This reaction occurs stepwise with the first conversion of triglycerides to diglycerides and then to monoglycerides and finally to glycerol. The complete transesterification of 1 mol of triglycerides requires 3 mol of alcohol, producing 1 mol of glycerol and 3 mol of fatty esters. Considering that the reaction is reversible, large excess of alcohol is used in industrial processes to 28 Chapter 1. Literature review and research aim ensure the direction of fatty acid esters (Fukuda et al., 2001). Methanol is the preferred alcohol for industrial use because of its low cost, although other alcohols like ethanol, propanol and butanol are also commonly used (Ataya et al., 2007). Figure 1.9 Transesterification of oil to biodiesel. R1-3 indicate hydrocarbon groups. In addition to heat, a catalyst is needed to facilitate the transesterification. The transesterification of triglycerides can be catalyzed by acids (Ataya et al., 2007; Guan et al., 2009; Miao et al., 2009; Furukawa et al., 2010), alkalis (Leung & Guo, 2006; Qian et al., 2008) or enzymes (Torres et al., 2005; Shah & Gupta, 2007; Su et al., 2007; Raita et al., 2010). Acid transesterification is considered suitable for the conversion of feedstocks with high free fatty acids but its reaction rate is low (Gerpen, 2005). In contrast, alkali-catalyzed transesterification has a much higher reaction rate, approximately 4000 times faster than the acid-catalyzed one (Fukuda et al., 2001). In this context, alkalis (sodium hydroxide and potassium hydroxide) are preferred as catalysts for industrial production of biodiesel. The production of biodiesel by alkali process is shown in Figure 1.10. The use of lipases as transesterification catalysts has also attracted much attention as it produces high purity product and enables easy separation from the byproduct glycerol (Ranganathan et al., 2008). However, the cost of enzyme is still relatively high and remains a barrier for its industrial implementation. In addition, it has been proposed that biodiesel can be prepared 29 Chapter 1. Literature review and research aim from oil via transesterification with supercritical methanol (Saka & Kusdiana, 2001; Demirbas, 2002). Table 1.3 shows the comparison of various methanolic transesterification methods, in terms of reaction temperature and time; while Table 1.4 summarizes the advantages and disadvantages of various transesterification methods. Oil Transesterification Alkali + Methanol Separation Evaporation of methanol Upper phase Waste water-alkali Washing Biodiesel Lower phase Evaporation of methanol Saponified products Purification Glycerol Figure 1.10 Production process of biodiesel using alkali as the catalyst (Ranganathan et al., 2008). 30 Chapter 1. Literature review and research aim Table 1.3 Comparison of various methanolic transesterification methods (Demirbas, 2008) Method Reaction temperature (K) Reaction time (min) Acid or alkali catalytic process 303-338 60-360 Boron trifluoride–methanol 360-390 20-50 Sodium methoxide–catalyzed 293-298 4-6 Non-catalytic supercritical methanol 523-573 6-12 Catalytic supercritical methanol 523-573 0.5-1.5 Table 1.4 Advantages and disadvantages of various transesterification methods (Huang et al., 2010) Type of Advantages Disadvantages Chemical Reaction condition can be well Reaction temperature is relative high catalysis controlled and the process is complex Large scale production The later disposal process is complex The cost of the production The process need much energy process is cheap Need a installation for methanol recycle The methanol produced in the The waste water pollutes the process can be recycled environment transesterification High conversion of the production Enzymatic Moderate reaction condition Limitation of enzyme in the conversion catalysis The small amount of methanol of short chain of fatty acids required in the reaction Chemicals exist in the process of Have no pollution to natural production are poisonous to enzyme environment Supercritical Easy to be controlled High temperature and pressure in the fluid techniques It is safe and fast reaction condition leads to high Friendly to environment production cost and wastes energy 31 Chapter 1. Literature review and research aim 1.3.3 Biodiesel feedstocks Biodiesel can be produced from a variety of feedstocks, including plant oils, animal fats and waste oils as well as algae (Demiras, 2008). Each feedstock has its advantages and disadvantages in terms of oil content, fatty acid composition, biomass yield and geographic distribution. Depending on the origin and quality of feedstocks, changes may be required for the production process of biodiesel. 1.3.3.1 Plant oils The use of plant oils as biodiesel feedstocks has been long recognized and well documented in numerous studies (Saka & Kusdiana, 2001; de Oliveira et al., 2005; Hill et al., 2006; Rashid & Anwar, 2008; Abdullah et al., 2009; Hawash et al., 2009; Graef et al., 2009; Sahoo & Das, 2009; Patil & Deng, 2009; Jain & Sharma, 2009; Nakpong & Wootthikanokkhan, 2010). These feedstocks include the oils from soybean, rapeseed, palm, canola, peanut, cottonseed, sunflower and safflower. Based on the geographic distribution, soybean is the primary source for biodiesel in USA, palm oil is used as a significant biodiesel feedstock in Malaysia and Indonesia, and rapeseed is the most common base oil used in Europe for biodiesel production (Demiras, 2008). The vast majority of these plants are also used for food and feed production, which means that possible food versus fuel conflicts are present. Thus, the use of these plant oils as feedstocks for biodiesel seems insignificant for the developing countries which are importers of edible oils (Meher et al., 2008). In addition to these edible oils, various non-edible, tree-borne oils from jatropha, karanja, jojoba and neem are the potential biodiesel feedstocks (Meher et al., 2008; Sahoo & Das, 2009; Jain & Sharma, 2009). Jatropha and karanja are two oilseed plants that are not widely exploited due to the presence of toxic components in the oils. In India, they are popularly used as 32 Chapter 1. Literature review and research aim biodiesel feedstocks. The physical properties of biodiesel from various plant feedstocks are listed in Table 1.5. Table 1.5 Physical properties of biodiesel from varous feestocks. Sources: Knothe, 2005; Meher et al., 2008 Feedstock; ester CN HG (kj/kg) KV (40 ℃; mm2/s) CP (℃) PP (℃) FP (℃) No. 2 Diesel 47.0 45343 2.7 -15.0 -33.0 52 Soybean; methyl 49.6 37372 4.18 -1.1 -3.9 190.6 Palm; ethyl 56.2 39070 4.50 (37.8 ℃) 8 6 — Rapeseed; methyl 47.9 39870 4.76 (37.8 ℃) -3 -9 166 Coconut; ethyl 67.4 38158 3.08 5 -3 190 Corn; methyl 65 38480 4.52 -3.4 -3 111 Sunflower; methyl 58 38472 4.39 1.5 3 110 Safflower; ehtyl 62.2 39872 4.31 -6 -6 178 Cottonseed; methyl 51.2 — 6.8 (21 ℃) — -4 110 Olive; methyl 61 37287 4.70 -2 -3 >110 Mustard; ethyl 54.9 40679 5.66 1 -15 183 Jatropha; methyl 51 — 4.84 — — 191 Karanja; methyl 56 — 4.77 — — 174 Tallow; methyl 61.8 37531 4.99 15.6 12.8 187.8 Grease; ethyl — — 6.20 5 -1 — Used frying oil; methyl 59 37337 4.50 1 -3 >110 Waste olive oil; methyl 58.7 — 5.29 -2 -6 — CN, cetane number; HG, gross heat of combustion; KV, kenematic viscosity; CP, cloud point; PP, pour point; FP, flash point. 33 Chapter 1. Literature review and research aim 1.3.3.2 Animal fats and waste oils In addition to the plant oils, animal fats and waste oils are the potential sources for commercial biodiesel production (Thompson et al., 2010). Among these feedstocks, tallow, lard, yellow grease and waste cooking oils have received most interest (Canakci, 2007; Phan & Phan, 2008; Banerjee et al., 2009; da Cunha et al., 2009; Dias et al., 2009; Diaz-Felix et al., 2009; Oner & Altun, 2009). However, animal fats and waste oils usually contain large amounts of free fatty acids, which can be as high as 41.8% (Canakci, 2007). Free fatty acids cannot be directly converted to biodiesel in alkali-catalyzed transesterificatoin but react with alkali to form soaps that inhibit the separation of biodiesel from glycerin and wash water fraction (Huang et al., 2010). A two-step process was developed for these high fatty acid feedstocks: acid-catalyzed pretreatment and alkali-catalyzed transesterificaton (Canacki & Van Gerpen, 2003). Because animal fats and waste oils have relatively high level of saturation (Canakci, 2007), the biodiesel from these sources exhibits poor cold flow properties (Table 1.5). 1.3.3.3 Algal oils Algae represent a wide variety of aquatic photosynthetic organisms with the potential of producing high biomass and accumulating high level of oil. The production of biodiesel from algal oil has long been recognized and been evaluated in response to the United States Department of Energy for research in alternative renewable energy (Sheehan et al., 1998). Currently, the commercialization of algae-derived biodiesel is still in its infancy stage. An unprecedentedly increasing interest was received, in terms of algal strain selection, biomass production, lipid yielding, transesterification technologies, fuel properties and engine tests (Miao & Wu, 2006; Xu et al., 2006; Li et al., 2007; Ross et al., 2008; Demirbas, 2009; Greenwell et al., 2009; Pruvost et al., 2009; Rodolfi et al., 2009; Yoo et al., 2009; Brennan & Owende, 2010; Xiong et al., 2010). Algae have 34 Chapter 1. Literature review and research aim been considered as the only feedstock of biodiesel that has the potential to displace fossil diesel (Chisti, 2007). 1.3.4 Potential and prospect of microalgal biodiesel Microalgae can grow autotrophically using CO2 from air and light through photosynthetic reactions and/or heterotrophically utilizing organic compounds as carbon and energy sources. Compared with higher plants, microalgae exhibit higher photosynthetic efficiency and growth rate (Chisti, 2007). Commonly, microalgae are cultivated photoautotrophically in open ponds or enclosed bioreactors for biomass production (Molina et al., 1999; Carvalho et al., 2006; Spolaore et al., 2006). A comparison of open and enclosed culture systems for microalgae is shown in Table 1.6. However, it is not easy to obtain high cell densities on a large scale in autotrophic culture systems because of light limitation or photoinhibition when exposed to high light (Chen, 1996; Harker et al., 1996; Wen et al., 2003). In contrast, heterotrophic culture provides several advantages over autotrophic culture, including good control of cultivation parameters, elimination of light requirement, high cell biomass obtained and low-cost for algal biomass harvest (Chen, 1996). Heterotrophic algal biomiass prodcution has been documented in many studies (Hata et al., 2001; Wen & Chen, 2001; Shi & Chen, 2002; Ip & Chen, 2005; Sun et al., 2008; Johnson & Wen, 2009; Liang et al., 2009). Chlorella protothecoides, a well-studied green microalga, is considered to be feasible for biodiesel feedstocks under heterotrophic culture conditions (Miao & Wu, 2004, 2006; Xu et al., 2006; Li et al., 2007; Gao et al., 2010; Xiong et al., 2010). 35 Chapter 1. Literature review and research aim Table 1.6 A comparison of open and closed culture systems for microalgae. Source: Mata et al., 2010 Culture systems Open ponds Enclosed bioreactors Contamination control Difficult Easy Contamination risk High Reduced Sterility None Achievable Process control Difficult Easy Species control Difficult Easy Mixing Very poor Uniform Operation regime Batch or Batch or semi-continuous semi-continuous Area/volume ration Low High Algal cell density Low High Investment Low Hight Operation cost Low High Light utilization efficiency Poor High Temperature control difficult More uniform temperature Productivity Low High Hydrodynamic stress on algae Very low Low-high Evaporation of growth medium High Low Gas transfer control Low High O2 inhibition < bioreactors Great problem Scale-up Difficult Difficult In addition to biomass, lipid content is another important factor to assess the potential of microalgae for biodiesel production. Over the past few decades, 36 Chapter 1. Literature review and research aim thousands of algae and cyanobacterial species have been screened for high lipid production, and numerous oleaginous species have been isolated and characterized. The lipid contents of these oleaginous algae are species- and/or strains-dependent and may vary greatly, as shown in Figure 1.11. Under optimal growth conditions, algae commonly synthesize a low content of lipids (i.e., averagely 25.5% of dry weight for green algae, Figure 1.11A), with membrane lipids (e.g., phospholipids and glycolidips) being the main components; whereas under unfavorable environmental or stress conditions, a great increase in total lipids was observed (e.g., averagely 45.7% of dry weight for green algae, Figure 1.11A) with neutral lipids in particular triacylglycerols (TAGs) being dominant (Hu, 2004). TAGs are considered to be superior to phospholipids or glycolipids for biodiesel feedstocks because of their higher percentage of fatty acids and lack of phosphate (Pruvost et al., 2009). Unlike higher plants in which individual classes of lipids may be synthesized and localized in a specific cell, tissue or organ, algae produce these different lipids in a single cell (Hu et al., 2008b). The synthesized TAGs are deposited in lipid bodies located in cytoplasm of algal cells (Rabbani et al., 1998; Damiani et al., 2010). 37 Chapter 1. Literature review and research aim Figure 1.11 Cellular lipid content in various classes of microalgae and cyanobacteria under normal growth and stress conditions. (A) Green microalgae; (B) diatoms; (C) oleaginous algae from other eukaryotic algal taxa; (D) cyanobacteria. Open circles: cellular lipid contents obtained under normal growth or nitrogen-replete conditions; closed circles: cellular lipid contents obtained under nitrogen-depleted or other stress conditions. The differences in cellular lipid content between cultures under normal growth and stress growth conditions were statistically significant for all three groups (A, B and C) of algae examined using Duncan’s multiple range test with the ANOVA procedure. Source: Hu et al., 2008b. The important properties of biodiesel such as cetane number, viscosity, cold flow, oxidative stability, are largely determined by the composition and structure of fatty acid esters which in turn are determined by the characteristics of fatty acids of biodiesel feedstocks, for exmaple carbon chain length and unsaturation degree (Knothe, 2005b). Fatty acids are either in saturated or unsaturated form, and the unsaturated fatty acids may vary in the number and position of double bones on the acyl chain. Based on the number of double bones, unsaturated fatty acids are clarified into monounsaturated fatty acids (MUFAs) and polyunsaturated fatty acids (PUFAs). The fatty acid profile of a great many algal species has been investigated and is shown in Table 1.7. The synthesized fatty acids in algae are commonly in medium length, ranging from 16 to 18 carbons, despite the great variation in fatty acid composition. Specifically, the major fatty acids are C16:0, C18:1 and C18:2 or C18:3 in green algae, C16:0 and C16:1 in diatoms and C16:0, C16:1, C18:1 and C18:2 in cyanobacteria. It is worthy to note that these data are obtained from algal species under specific conditions and vary greatly when algal cells are exposed to different environmental or nutritional conditions such as temperature, pH, or nitrogen concentration (Wada & Murata, 1990; Chen & Johns, 1991; Tatsuzawa & 38 Chapter 1. Literature review and research aim Takizawa, 1996; Khozin-Goldberg et al., 2002; Liu et al., 2005). Generally, saturated fatty esters possess high cetane number and superior oxidative stability; whereas unsaturated, especially polyunsaturated, fatty esters have improved low-temperature properties (Knothe, 2008). In this regard, it is suggested that the modification of fatty esters, for example the enhanced proportion of oleic acid (C18:1) ester, can provide a compromise solution between oxidative stability and low-temperature properties and therefore promote the quality of biodiesel (Knothe, 2008, 2009). Thus, microalgae with high oleic acid are suitable for biodiesel production. Currently the commercial production of biodiesel is mainly from plant oils and animal fats. However, the plant oil derived biodiesel cannot realistically meet the demand of transport fuels because large arable lands are required for cultivation of oil plants, as demonstrated in Table 1.8. Based on the oil yield of different plants, the cropping area needed is calculated and expressed as a percentage of the total U.S. cropping area. If soybean, the popular oil crop in United States is used for biodiesel production to meet the existing transport fuel need, 5.2 times of U.S. cropland will need to be employed. Even the high-yielding oil plant palm is planted as the biodiesel feedstock, more than 50% of current U.S. arable lands have to be occupied. The requirement of huge arable lands and the resulted conflicts between food and oil make the biodiesel from plant oils unrealistic to completely replace the petroleum derived diesel in the foreseeable future. It is another case, however, if microalgae are used to produce biodiesel. As compared with the conventional oil plants, microalgae possess significant advantages in biomass production and oil yield and therefore the biodiesel productivity. In terms of land use, microalgae need much less than oil plants, thus eliminating the competition with food for arable lands (Table 1.8). Based on the above mentioned information, microalgae appear to be the only source of biodiesel that has the potential to replace fossil derived diesel. 39 Chapter 1. Literature review and research aim Table 1.7 Fatty acid composition of some algal species (% of total fatty acids) Fatty C14:0 C16:0 C16:1 C16:2 C16:3 C17:0 C18:0 C18:1 C18:2 C18:3 C18:4 C18:5 C20:0 C20:1 C20:4 C20:5 C22:5 C22:6 acids Chlorophyta C.p. 1.31 12.94 0.89 2.76 60.84 17.28 0.35 0.42 C.v. 18.0 5.0 12.0 2.1 9.2 43.0 10.0 C.s. 25.4 3.1 10.7 4.1 1.4 12.4 34.4 7.1 H.p.a 1.25 22.49 0.64 0.19 3.15 19.36 26.9 17.04 0.2 0.13 0.89 0.57 C.r. 4.0 36.1 1.8 4.4 13.3 17.8 20.5 2.1 C.sp 2.0 39.5 2.1 1.5 30.2 2.7 22.1 P.i. 9.1 0.7 0.6 2.1 15.1 9.3 1.6 1.2 58.9 Bacillariophyta N.l. 16.9 28.5 23.9 0.7 5.1 3.4 4.1 5.0 11.7 Ch.sp 23.6 9.2 36.5 6.9 2.6 2.0 3.0 1.4 0.6 4.1 8.0 1.0 B.a. 32.0 5.0 27.0 2.0 8.0 26.0 Cyanophyta Syn. 52.0 3.0 1.0 3.0 9.0 29.0 3.0 N.f. 0.65 21.27 14.91 6.2 22.59 15.03 19.35 N.c.b 23.5 22.5 5.6 21.1 14.1 Others G.c. 22.0 4.4 4.0 6.6 3.9 5.5 39.2 13.3 N.sp 6.9 19.9 27.4 1.7 3.5 0.7 4.2 34.9 S.sp 3.2 9.4 0.7 0.5 1.5 1.5 5.4 10.6 43.1 1.8 0.1 18.8 Abbreviation of algal species: C.p., Chlorella protothecoides (Li et al., 2007); C.v., Chlorella vulgaris (Harris et al., 1965); C.s., Chlorella sorokiniana (Chen & Johns, 1991); H.p., Haematococcus pluvialis (Damiani et al., 2010); C.r. and C.sp, Chlamydomonas reinhardtii and Chlamydomonas sp. (Tatsuzawa and Takizawa, 1996); P.i., Parietochloris incise (Khozin-Goldberg et al., 2002); N.l., Nitzschia laevis (Chen, 2007); Ch.sp, Chaetoceros sp. (Renaud et al., 2002); B.a., Biddulphia aurica (Orcuut & Patterson, 1975); Syn., Synechocystis PCC6803 (Wada & Murata, 1990); N.f., Nostoc flagelliforme (Liu et al., 2005); N.c., Nostoc commune (Pushparaj et al., 2008); G.c., Glossomastrix chrysoplasta (Kawachi et al., 2002); N.sp, Nannochloropsis sp (Sukenik, 1999); S.sp, Scrippsiella sp (Mansour et al., 1999). a from neutral lipids, some short-chain fatty acids in low content are not listed. b unknown fatty acids are not listed. 40 Chapter 1. Literature review and research aim Table 1.8 Comparation of microaglae with other biodiesel feedstocks. Source: Chisti, 2007; Mata et al., 2010 Plant source Oil yeild Land area needed Percentage of existing (L/ha year) (M ha) a US cropping areaa Corn 172 3480 1912 Hemp 363 1650 906 Soybean 636 940 516 Jatropha 741 807 443 Camelina 915 650 357 Canola 974 610 335 Sunflower 1070 560 307 Castor 1307 450 247 Palm oil 5366 110 60.4 Microalgae b 58,700 9.0 4.9 Microalgae c 97,800 5.4 3.0 Microalgae d 136,900 3.9 2.1 a for meeting all transport fuel needs of the United States. b 30% oil by dry weight. c 50% oil by dry weight. c 70% oil by dry weight. However, the cost of microalgal biodiesel still remains high, which is mainly attributed to the high cost of microalgal oil. The microalgal oil is estimated to cost $2.8/L, much higher than that of crude fossil oil and plant oil (Chisti, 2007). To make algae-derived biodiesel cost-competitive with petroleum, microalgal oil should be at a price like this: Calgal oil = 6.9 × 10-3 Cpetroleum, where Calgal oil ($ per liter) is the price of microalgal oil and Cpetroleum ($ per barrel) is the price of crude fossil oil (Chisti, 2007). For example, if the price of crude oil approaches to $100/barrel, it appears to be economical for microalgal oil to replace crude petroleum at the cost of $0.69/L. Production cost of microalgal biodiesel may be brought down substantially by improving capabilities of 41 Chapter 1. Literature review and research aim microalgae through metabolic and genetic engineering or by using a biorefinery based production strategy. 1.3.5 Lipid metabolism in microalgae Although lipid metabolism, in particular the biosynthesis of fatty acids and TAGs, is poorly understood in algae, it is generally considered that the basic pathways for fatty acid and TAGs biosynthesis are similar to those demonstrated in higher plants. 1.3.5.1 Fatty acid/lipid biosynthesis Similar to plants, microalgae synthesize fatty acids in the chloroplast using a single set of enzymes. A simplified schedule for fatty acid biosynthesis is shown in Figure 1.12. The formation of malonyl CoA from acetyl CoA is generally regarded as the first reaction of the fatty acid biosynthetic pathway, which is catalyzed by acetyl CoA carboxylase (ACCase). The malonyl group of malonyl CoA is transferred to a protein co-factor on the acyl carrier protein (ACP), resulting in the formation of malonyl ACP that involves in subsequent condensation and elongation reactions. The first condensation reaction is catalyzed by 3-ketoayl ACP synthase III (KAS III), forming a four-carbon product. KAS I and KAS II catalyze the subsequent condensations and finally the saturated 16:0- and 18:0-ACP are produced. To produce unsaturated fatty acids, the double bonds are introduced by the enzyme stearoyl ACP desaturase (SAD). Unlike plants, some microalgae produce long-chain acyl ACPs (C20-C22) that derive from the further elongation and/or desaturation of C18 (Figure 1.13). The final fatty acid composition of individual algae is determined by the activities of enzymes that use these acyl ACPs as substrates at the termination phase of fatty acid biosynthesis. These fatty acids are then used as the precursors for the synthesis of 42 Chapter 1. Literature review and research aim cellular membranes and neutral storage lipids like TAGs. Acetyl-CoA ACCase KAS III Malonyl-CoA C4:0-ACP Chloroplast KAS I C16:0-ACP KAS II C18:0-ACP SAD C18:1-ACP C16-C18-CoA Figure 1.12 A simplified schedule for fatty acid biosynthesis (Ohlrogge & Jaworski, 1997). It has been proposed that the biosynthesis of TAG occurs in cytosol via the direct glycerol pathway (Figure 1.14). Generally, acyl-CoAs sequentially react with the hydroxyl groups in glycerol-3-phosphate to form phosphatidic acid. These two reactions are catalyzed by glycerol-3-phospate acyl transferase and lysophosphatidic acid acyl transferase respectively. Dephosphorylation of phosphatidic acid results in the release of diacylglycerol which accepts a third acyl from CoA to form TAG. This final step is catalyzed by diacylglycerol acyltransferase, an enzymatic reaction that is unique to TAG synthesis. In addition, an alternative pathway that is independent of acyl-CoA may also be present in 43 Chapter 1. Literature review and research aim microalgae for TAG biosynthesis (Dahlqvist et al., 2000). This pathway employs phospholipids as acyl donors and diacylglycerols as the acceptors and might be activated when algal cells are exposed to stress conditions because algae usually undergo rapid degradation of the photosynthetic membranes and concurrent accumulation of cytosolic TAG-enriched lipid bodies (Hu et al., 2008b). C18:0 △ 9 desaturase C18:1 △ 12 desaturase C18:2 (n-6) △ 15 desaturase △ 9, 12 C18:3 (n-3) △ 9, 12, 15 C20:2 (n-6) △ 11, 14 C18:3 (n-6) △ 15 desaturase △ 6, 9, 12 C18:4 (n-3) △ 6, 9, 12, 15 △ 8 desaturase C20:3 (n-6) △ 17 desaturase C20:4 (n-3) △ 8, 11, 14 △ 8, 11, 14, 17 Elongase △ 6 desaturase △ 6 desaturase Elongase Elongase C20:3 (n-3) △ 11, 14, 17 Elongase △ 5 desaturase △ 8 desaturase △ 5 desaturase C20:4 (n-6) △ 17 desaturase C20:5 (n-3) △ 5, 8, 11, 14 △ 5, 8, 11, 14, 17 Elongase Elongase C22:4 (n-6) △ 7, 10, 13, 16 C22:5 (n-3) △ 7, 10, 13, 16, 19 C22:5 (n-6) △ 4, 7, 10, 13, 16 C22:6 (n-3) △ 4, 7, 10, 13, 16, 19 △ 4 desaturase △ 4 desaturase Figure 1.13 The elongation and desaturation of acyl chain of fatty acids (Guschina & Harwood, 2006). 44 Chapter 1. Literature review and research aim Figure 1.14 The biosynthesis of TAG in microalgae (Huang et al., 2010) 1.3.5.2 Factors affecting lipid accumulation and fatty acid composition The lipid content and fatty acid composition are species/strain-specific and can be greatly affected by chemical and physical stimuli. The main chemical stimuli are nutrient concentration, salinity and pH of medium and the main physical stimuli are light intensity and temperature. 1.3.5.2.1 Nutrients Of all the nutrients surveyed, nitrogen is the most critical one affecting lipid metabolism in algae. The influence of nitrogen source and concentration on lipid and fatty acid production has been investigated in a number of microalgae (Takagi et al., 2000; Li et al., 2005; Li et al., 2008a; Solovchenko et al., 2008; Hsieh & Wu, 2009; Pruvost et al., 2009; Li et al., 2010). Nitrate was suggested to 45 Chapter 1. Literature review and research aim be superior to other nitrogen sources such as urea and ammonium for algal lipid production (Li et al., 2008a). Generally, low concentration of nitrogen in the medium favors the accumulation of lipids particularly TAGs and total fatty acids. But in some cases, nitrogen starvation caused decreased synthesis of lipids and fatty acids (Saha et al., 2003). As for the effect of nitrogen on fatty acid composition, there is no general trend available and it appears to be species/strain dependent. For example, in cyanobacteria, increased levels of C16:0 and C18:1 and decreased C18:2 level were observed in response to nitrogen deprivation (Piorreck & Pohl, 1984). In the marine alga Pavlova viridis, nitrogen depletion resulted in an increase in saturated, monounsaturated fatty acids and C22:6 (n-3) contents (Li et al., 2005). Nitrogen starvation brought about a strong increase in the proportion of C20:4 (n-6) in the green algal Parietochloris incisa (Solovchenko et al., 2008). Other types of nutrient that affect lipid production include phosphorus, silicon, sulfur and iron. In response to phosphorus deprivation, the enhanced accumulation of lipids was observed in Ulva pertusa, Scenedesmus sp.LX1, Monodus subterraenus, Phaeodactylum tricornutum, Chaetoceros sp., Isochrysis galbana and Pavlova lutheri (Reitan et al., 1994; Floreto et al., 1996; Khozin-Goldberg & Cohen, 2006; Li et al., 2010), while reduced lipid production was demonstrated in Nannochloris atomus and Tetraselmis sp. (Reitan et al., 1994). Among the marine species surveyed by Reitan et al. (1994), phosphorus deprivation caused increased contents of C16:0 and C18:1 and decreased polyunsaturated fatty acids; whereas in U. pertusa, phosphorus deprivation decreased the proportion of C16:0 (Floreto et al., 1996). Silicon is the key nutrient for lipid metabolism of diatoms. The deficiency of silicon increased the accumulation of neutral lipids, as well as of saturated and monounsaturated fatty acids in Cyclotella cryptic (Roessler, 1988). Studies also suggested that sulfur limitation could promote lipid production in microalgae (Otsuka, 1960; Sato et al., 2000). In addition, iron at a relative high concentration could induce the accumulation of lipids in the green alga Chlorella vulgaris (Liu et al., 2008). 46 Chapter 1. Literature review and research aim 1.3.5.2.2 Light Light has a marked effect on the lipid production and fatty acid composition in microalgae (Nichols, 1965; Koskimies-Soininena & Nyberg, 1987; Sukenik et al., 1989; Napolitano, 1994; Brown et al., 1996; Zhekisheva et al., 2002, 2005; Khotimchenko & Yakovleva, 2005; Damiani et al., 2010). Generally, low light intensity favors the formation of polar lipids such as the membrane lipids associated with the chloroplast; whereas high light intensity benefits the accumulation of neutral storage lipids in particular TAGs. In H. pluvialis, for example, high light resulted in a great increase of both neutral and polar lipids, but the increase extent of neutral lipids was much greater than that of polar lipids, leading to the dominant proportion of neutral lipids in the total lipids (Zhekisheva et al., 2002, 2005). Although the effect of light intensity on fatty acid composition differs among the algal species and/or strains, there seems be a general trend, with a few exceptions, that the increase of light intensity contributes to the enhanced proportions of saturated and monounsaturated fatty acids and the concurrently the reduced proportion of polyunsaturated fatty acids (Sukenik et al., 1989; Zhekisheva et al., 2002, 2005; Eabregas et al., 2004). 1.3.5.2.3 Temperature Temperature can affect lipid and fatty acid composition of many organisms including algae (Sato & Murata, 1980; Lynch & Thompson, 1982; Henderson & Mackinlay, 1989; Wada & Murata, 1990; Thompson et al., 1992; Renaud et al., 1995, 2002). In response to temperature shift, algae commonly alter the physical properties and thermal responses of membrane lipids to maintain fluidity and function of membranes (Somerville, 1995). In general, increased temperature causes increased fatty acid saturation and at the same time decreased fatty acid unsaturation. For example, C14:0, C16:0, C18:0 and C18:2 increased and C18:3 (n-3), C18:4, C20:5 and C22:6 decreased in Rhodomonas sp., and 47 Chapter 1. Literature review and research aim C16:0 increased and C18:4 decreased in Cryptomonas sp. when temperature increased (Renaud et al., 2002). As for the effect of temperature on lipids, it differs with a species-dependent manner. In response to increasing temperature, increased lipid content was observed in Nanochloropsis salina (Boussiba et al., 1987) and Ochromonas danica (Aaronson, 1973); decreased lipid content was observed in Chroomonas salina (Henderson & Mackinlay, 1989), Nitzschia paleacea (Renaud et al., 1995) and Chaetoceros sp. (Renaud et al., 2002); while in Chlorella sorokiniana (Patterson, 1970) and Isochrysis sp. (T.ISO) (Renaud et al., 2002), lipid content showed no significant change. 1.3.5.2.4 Salinity Salinity stress can influence cell membrane permeability and fluidity. Cells adapt themselves to high salinity through the compositional changes in sterols and polar lipids (Parida & Das, 2005). The effect of salinity on lipid profile and fatty acid composition was reported in some species of microalgae such as Botryococcus braunii, Chaetoceros cf. wighamii, Cladophora vagabunda, Crypthecodinium cohnii, Dunaliella tertiolecta, Nitzschia laevis (Elenkov et al., 1996; de Castro Araujo & Garcia, 2005; Takagi et al., 2006; Rao et al., 2007; Chen et al., 2008). Generally, salinity stress favors the accumulation of lipids and causes enhanced fatty acid saturation and reduced fatty acid unsaturation in microalgae. 1.4 The green alga Chlorella zofingiensis The green algae (Chlorophyta) are named for their typical color, the bright grass-green characteristic of land plants. They are a large group of algae consisting of more than 6000 species distributed in different habitats (Thomas, 2002). Most of green algae live in fresh water, with a minor proportion locate in 48 Chapter 1. Literature review and research aim marine water, damp soil, land plants or even snow and ice (Lee, 2008). Green algae exhibit a variety of morphologies. They can be motile or no-motile, unicellular or colonial flagellates, usually but not always with two flagella per cell (Wikipedia, 2010). C. zofingiensis is a fresh water microalga belonging to Class Chlorophyceae, Order Chlorococcales and Family Chlorellaceae (Pickett-Heaps, 1975). C. zofingiensis cells are non-motile and in unicellular and spherical form, with the cell size ranging from 2 μm to 15 μm in diameter. Through the formation of autospores, C. zofingiensis asexually reproduces daughter cells from non-motile parental cells (Lee, 2008). C. zofingiensis can grow well photoautotrophically, heterotrophically and mixotrophically (Orosa et al., 2000, 2001; Ip et al., 2004; Ip & Chen 2005; Sun et al. 2008). Under optimal growth conditions, C. zofingiensis accumulated mainly chlorophylls and primary carotenoids such as lutein; while under stress conditions like high light, nitrogen starvation, or high carbon/nitrogen ratio in the dark, C. zofingiensis predominantly produced astaxanthin and thus exhibited a deep red color (Orosa et al., 2001; Ip & Chen, 2005). So far, studies about C. zofingiensis have primarily focused on astaxanthin production, while the investigations about lipids and fatty acids have been rarely touched. 1.4.1 Pigment profiles Both primary and secondary carotenoids have been found in C. zofingiensis. Like plants and other algae, C. zofingiensis synthesizes and accumulates primary carotenoids (e.g., β-carotene, lutein and zeaxanthin) in chloroplast (Rise et al., 1994; Grunewald et al., 2001). In contrast, secondary carotenoids such as astaxanthin, canthaxanthin and adonixanthin are found to accumulate in lipid bodies outside the chloroplast (Rise et al., 1994; Del Campo et al., 2004). Commonly, the accumulation of secondary carotenoids is considered 49 Chapter 1. Literature review and research aim related to the stress conditions, under which algal cells get protected against oxidative damages by these anti-oxidative carotenoids through quenching the excessive ROS and other free radicals (Rise et al., 1994; Bar et al., 1995). For example, when growing under high light and nitrogen starvation, C. zofingiensis produced mainly secondary carotenoids, with around 70% being astaxanthin (Rise et al., 1994). The photoautotrophic cultivation of C. zofingiensis generally gives a low cell biomass and thus a low astaxanthin yield. For example, as reported by Orosa et al. (2000), the cell biomass and carotenoid concentrations in photoautotrophic culture were limited to 0.72 g L-1 and 2.81 mg L-1 , respectively. Furthermore, the attenuated light absorption caused by mutual shading of cells in large-scale cultures can severely affect the productivity of algal biomass and products (Chen, 1996). To overcome such problems, mixotrophy and heterotrophy have been employed for C. zofingiensis growth and astaxanthin production (Ip et al., 2004; Ip & Chen, 2005). In heterotrophic culture, C. zofingiensis utilized organic substrate (e.g., glucose) as the sole energy and carbon source and achieved a much higher cell density as compared with in photoautotrophic culture (Orosa et al., 2000; Ip et al., 2004). Ip (2005) intensively investigated the heterotrophic production potential of astaxanthin by Chlorella zofingiensis: (1) a high initial carbon to nitrogen ratio (e.g., 180) was found to enhance astaxanthin biosynthesis in algal cells; (2) through the manipulation of the medium nutrients and cultivation environments, the maximal yield of astaxanthin (9.9 g L-1) was obtained when the algal cells were grown on the medium consisting of 0.44 g L-1 nitrate, 0.16 g L-1 phosphate and 8 mg L-1 ferrous ion at 30 and pH 5.5; (3) external supplementation of ROS or reactive nitrogen species/reactive nitrogen intermediate further induced astaxanthin synthesis in the algal cells; (4) endogenous arousal of oxidative stresses caused by salt or by inhibition of antioxidative enzymes also triggered increased astaxanthin production. By using a well designed fed-batch fermentation strategy, up to 32.4 mg L-1 astaxanthin was obtained in the algal cells, indicating the potential of employment of heterotrophic 50 Chapter 1. Literature review and research aim C. zofingiensis cells for commercial production of astaxanthin on a large scale (Sun et al., 2008). A general pigment profile of C. zofingiensis under heterotrophic conditions is shown in Figure 1.15. Astaxanthin occurred predominantly in the form of mono- and di-esters. 51 Chapter 1. Literature review and research aim 0.020 12 13 21 18 24 25 AU 0.015 0.010 32 16 1 27 26 20 0.005 3 9 2 14 5 6 10 78 11 10.00 11.00 4 15 19 17 37 22 23 28 30 33 29 31 34 38 35 36 39 0.000 8.00 9.00 12.00 13.00 14.00 15.00 16.00 17.00 18.00 Minutes 19.00 20.00 21.00 22.00 23.00 24.00 25.00 26.00 27.00 Figure 1.15 A HPLC chromatogram showing a general pigment profile of C. zofingiensis under heterotrophic conditions. 1 Neoxanthin; 2 Violaxanthin; 3 Antheraxanthin; 4,5,7,8,28,29,30,35,36 Unknown or degraded lutein and chlorophylls; 6 Astaxanthin; 9,10 Adonixanthin; 11 Phoenicoxanthin (adonirubin); 12 Lutein; 13,14,15 Zeaxanthin; 16,17 Canthaxanthin; 18 Chlorophyll b; 19 Hydroxyechinenone; 20,22 β−Cryptoxanthin; 21 Chlorophyll a; 23 Echinenone; 24,26 Astaxanthin mono-ester; 25,27 Adonixanthin mono-ester; 31 α−Carotene; 32,33,34 β−Carotene; 37,39 Astaxanthin di-ester; 38 Adonixanthin di-ester. Source: Sun, 2009. 52 Chapter 1. Literature review and research aim 1.4.2 Astaxanthin biosynthesis β-carotene is considered to be the major precursor for astaxanthin. Astaxanthin is biosynthesized from β-carotene via two proposed pathways: one starting from the oxygenation and then the hydroxylation producing the intermediates of echinenone, canthaxanthin and adonirubin, and the other from the hydroxylation and then the oxygenation via β-cryptoxanthin, zeaxanthin and adonixanthin as intermediates (Figure 1.16). It has been demonstrated that astaxanthin biosynthesis in the green alga H. pluvialis followed the pathway as indicated by solid dash in Figure 1.16 (Breitenbach et al., 1996; Fraser et al., 1998; Linden, 1999). In C. zofingiensis, however, there appears to be another case. The ketolase from H. pluvialis can efficiently convert canthaxanthin to astaxanthin, but shows a poor ability in conversion of zeaxanthin to astaxanthin (Fraser et al., 1997, 1998). In contrast, the C. zofingiensis ketolase exhibits a bi-functional activity: efficient introduction of keto groups to both β-carotene and zeaxanthin (Huang et al., 2006). Considering that substantial canthaxanthin (around 30% of total ketocarotenoids) accumulated and might represent the end product of oxygenated β-carotene in C. zofingiensis, the carotenoid hydroxylase from this alga might not accept canthaxanthin as a substrate (Rise et al., 1994). Based on the above results as well as the accumulated adonixanthin in both transformed E. coli and induced C. zofingiensis cells, a possible astaxanthin biosynthetic pathway different from that in H. pluvialis was proposed as indicated by dash arrow in Figure 1.16: oxygenation of zeaxanthin rather than hydroxylation of canthaxanthin (Huang et al., 2006; Wang & Chen, 2008). 53 Chapter 1. Literature review and research aim Phytoene PDS ζ-carotene β-carotene BKT CHY β-cryptoxanthin Echinenone CHY BKT Zeaxanthin Canthaxanthin BKT CHY Adonirubin Adonixanthin BKT CHY Astaxanthin Figure 1.16 Proposed pathways of astaxanthin biosynthesis in algae. PDS, phytoene desaturase; BKT, carotenoid ketolase; CHY, carotenoid hydroxylase. Adapted from Huang et al. (2006). Although high light, salinity and glucose are known to enhance the biosynthesis of astaxanthin in C. zofingiensis, the underlying mechanism of their reaction remains largely unknown at molecular level before the availability of genes involved in astaxanthin biosynthesis (Bar et al., 1995; Ip, 2005). Certain key genes, namely phytoene desaturase (PDS), carotenoid ketolase (BKT) and carotenoid hydroxylase (CHYb) have been isolated and characterized from C. zofingiensis recently (Huang et al., 2006, 2008). High light up-regulates the transcripts of PDS, CHYb, and BKT and greatly enhances the biosynthesis of zeaxanthin, canthaxanthin, and astaxanthin; while salinity stress only up-regulates 54 Chapter 1. Literature review and research aim the transcript of BKT and enhances the biosynthesis of canthaxanthin and astaxanthin (Li et al., 2009). This discrepancy might result from the generation of different intracellular ROS, which stimulated the up-regulation of specific carotenoid genes (Ip, 2005; Li et al., 2009). In dark-grown C. zofingiensis, glucose sensing (phosphorylation of glucose) directly up-regulates the transcription of CHYb, while the mitochondrial alternative pathway is closely related to the regulation of BKT, through which the biosynthesis of astaxanthin is regulated (Li et al., 2008b). Moreover, the involvement of transcriptional control on the carotenoid biosynthesis in C. zofingiensis by PDS, CHYb and BKT is suggested (Li et al., 2008b, 2009; Liu et al., 2010). Taken together, the availability of carotenoid genes, the demonstrated astaxanthin biosynthetic pathway and the possible mechanism of astaxanthin regulation provide a solid foundation for the genetic engineering of C. zofingiensis with an attempt to enhance astaxanthin production. 1.4.3 Lipid and fatty acid profiles Some Chlorella species such as C. vulgaris and C. protothecoides have been analyzed in terms of lipids and fatty acids (Miao & Wu, 2004; Cleber Bertoldi et al., 2006; Liu et al., 2008). No such information so far, however, is available for C. zofingiensis. Considering its fast growth and versatile cultivation modes, C. zofingiensis may have the potential as a biodiesel feedstock candidate, which will require further investigations, for example the lipid and fatty acid analyses. 1.5 Research aim C. zofingiensis represents a fast-growing green microalga that can grow under photoautotrophic, mixotrophic and heterotrophic conditions. It shows great potential as a source of the high-value carotenoid astaxanthin and may also be a 55 Chapter 1. Literature review and research aim potential source of lipids for biodiesel production. However, the relatively low cellular content of astaxanthin and the shortage of information about lipid and fatty acid profiles in C. zofingiensis hamper its commercial application. So the aim of this research was to improve the cellular accumulation of astaxanthin in C. zofingiensis and to assess the potential use of this alga as a biodiesel feedstock. Following shows the major work of my study: (1) The PDS gene from C. zofingiensis was isolated and characterized. (2) C. zofingiensis mutants with enhanced astaxanthin accumulation were generated by treatment of chemical mutagens coupled with norflurazon selection. (3) The molecular characterization of one mutant E17 was conducted. (4) The transformation of C. zofingiensis with a mutated PDS gene from E17 and the analysis of obtained transformants with promoted astaxanthin accumulation were performed. (5) The lipid and fatty acid profiles of photoautotrophic and heterotrophic C. zofingiensis were compared. (6) The lipid production and fatty acid profile of C. zofingiensis cultured in the dark with various carbon sources were investigated. (7) The optimized conditions for fatty acid production by heterotrophic C. zofingiensis were established. (8) The key genes BC and SAD involved in fatty acid biosynthesis were isolated and characterized from C. zofingiensis. 56 PART II GENETIC ENGINEERING OF CHLORELLA ZOFINGIENSIS FOR ENHANCED ASTAXANTHIN PRODUCTION 57 Chpater 2. Isolation and characterization of the phytoene desaturase gene from Chlorella zofingiensis Chapter 2 Isolation and characterization of the phytoene desaturase gene from Chlorella zofingiensis 2.1 Abstract Phytoene desaturase (PDS) is a rate-limiting enzyme in carotenoid biosynthesis. Algal PDS is inhibited by some herbicides, leading to the bleaching of cells due to the destruction of chlorophylls. Specific point mutations in PDS confer resistance to the herbicide norflurazon, suggesting that mutated PDS could be used as a dominant selectable marker for genetic engineering of algae, for which very few selectable markers are available. In this chapter, the isolation and characterization of the PDS gene from Chlorella zofingiensis were reported. The open reading frame of this PDS gene, interrupted by six introns, encoded a polypeptide of 558 amino acid residues. The deduced protein sequence showed significant homology with phytoene desaturases from other algae, cyanobacteria and higher plants. Expression of the PDS gene in Escherichia coli demonstrated that the enzyme was able to convert phytoene to ζ-carotene. The PDS gene in C. zofingiensis was shown to be up-regulated by high light and glucose treatment. With a single amino acid change (L516R), the mutated PDS exhibited 35-fold greater resistance to norflurazon but also a lower desaturation activity than the unaltered enzyme. The results indicated that certain point mutation could make Chlorella PDS herbicide-resistant and potentially useful in genetic engineering of C. zofingiensis. 2.2 Introduction As the biosynthesis of astaxanthin is observed only in a limited number 58 Chpater 2. Isolation and characterization of the phytoene desaturase gene from Chlorella zofingiensis of organisms (e.g., some marine bacteria, the red yeast Xanthophyllomyces dendrorhous, and some green algae) (Johnson & Schroeder, 1995), potential production of astaxanthin from microorganisms and transgenic plants has been the subject of intensive investigations in recent years (Gong & Chen, 1997; Mann et al., 2000; Stalberg et al., 2003; Ip & Chen, 2005; Steinbrenner & Sandmann, 2006). The unicellular green alga Haematococcus pluvialis has the highest astaxanthin accumulation (up to 4% of dry biomass) (Boussiba, 2000). However, its slow growth rate restricts its application. The green microalga Chlorella zofingiensis is a promising host for boosting astaxanthin production by metabolic engineering because the alga can grow fast (with a specific growth rate of over 0.031 h-1) and produce astaxanthin (with a astaxanthin yield up to 10.3 mg L-1) in the dark with glucose as sole carbon and energy source (Ip & Chen, 2005). Moreover C. zofingiensis has a similar mechanism of storing astaxanthin as H. pluvialis, suggesting that C. zofingiensis might be genetically modified to accumulate much higher amounts of astaxanthin. While it is now relatively easy to generate a transgenic plant, there are still significant technical challenges to develop functional transgenic systems for many commercially important algae. Endogenous promoters and terminators proved critical for the expression of heterologous genes in algae (Cerutti et al., 1997; Ohresser et al., 1997; Poulsen & Kroger, 2005; Steinbrenner & Sandmann, 2006). Thus, in order to improve the astaxanthin production in C. zofingiensis by genetic engineering, it is critical that an efficient selectable marker be developed. Phytoene desaturase (PDS) is the rate-limiting enzyme in carotenoid biosynthesis (Chamovitz et al., 1993). The plant and algal PDS carries out the first two-step desaturation of phytoene leading to the formation of ζ-carotene (Pecker et al., 1992; Sandmann, 1994). PDS is inhibited by some herbicides, which causes chlorophyll destruction and thus the cell bleaching. However, certain point-mutations in PDS were found to confer resistance to the herbicide norflurazon (Chamovitz et al., 1993; Arias et al., 2006). Thus, an endogenous PDS gene could be modified and used as a dominant selectable marker for nuclear transformation of commercially important algae for which stable transformation is still problematic. Plant and algal PDS genes are highly conserved and have similar catalytic properties (Pecker et al., 1992; Linden et al., 1995). The aim of the 59 Chpater 2. Isolation and characterization of the phytoene desaturase gene from Chlorella zofingiensis present study is to isolate and characterize the PDS gene from C. zofingensis. In addition, a modified PDS gene resistant to norflurazon was generated by site-directed mutagenesis. The obtained herbicide-resistant PDS gene could be very useful for genetic engineering of carotenoid biosynthesis in C. zofingiensis. 2.3 Materials and methods 2.3.1 Algal strain and culture conditions The green microalga C. zofingiensis (ATCC 30412) was obtained from the American Type Culture Collection (ATCC, Rockville, MD, USA). This algal strain was maintained and cultured at 25 °C under continuous illumination of 25 µmol photon m-2 s-1 in Kuhl medium (Kuhl & Lorenzen, 1964) consisting of (per liter) 1.01 g KNO3; 0.62 g NaH2PO4∙H2O; 0.089 g Na2HPO4∙2H2O; 0.247g MgSO4∙7H2O; 14.7 mg CaCl2∙2H2O; 6.95 mg FeSO4∙7H2O; 0.061 mg H3BO3; 0.169 mg MnSO4∙H2O; 0.287 mg ZnSO4∙7H2O; 0.0025 mg CuSO4∙5H2O; and 0.01235 mg (NH4)6MO7O24∙4H2O. The pH of the medium was adjusted to pH 6.5 prior to autoclaving. To investigate the effect of high light and glucose on expression of the PDS gene, photoautotrophically grown cells in exponential growth phase were exposed to continuous high light (120 μmol photon m-2 s-1) or provided with glucose (50 mM) in the dark for 0 to 48 h. 2.3.2 Genomic DNA and RNA isolation DNA was extracted using a modified cetyltrimethylammonim bromide (CTAB) method (Stewart & Via, 1993). RNA was isolated from aliquots of about 108 cells using the TriPure isolation reagent (Roche, Mannheim, Germany) according to the manufacturer’s manual. The concentration of DNA and total RNA was determined spectrophotometrically at 260 nm. 60 Chpater 2. Isolation and characterization of the phytoene desaturase gene from Chlorella zofingiensis 2.3.3 Cloning of PDS cDNA and its corresponding gene Degenerate primers dF and dR were designed based on the conserved amino acid sequences (GKVAAWK and LQWKEHS, respectively) of the PDS proteins from Chlamydomonas reinhardtii, H. pluvialis, Synechococcus sp. PCC 7942, Dunaliella salina and Arabidopsis thaliana (Chamovitz et al., 1991; Scolnik & Bartley, 1993; Harker & Hirschberg, 1997; McCarthy et al., 2004; Zhu et al., 2005). They were used for the amplification of a partial PDS cDNA from C. zofingiensis, which would provide sequence information for designing specific primers for rapid amplification of 5′ and 3′ cDNA ends (RACE). The primer sets used in this study are listed in Table 2.1. RACE was performed using the method described by Huang and Chen (2006). Genomic walking of the PDS gene was performed according to the approach described in the Universal GenomeWalker kit (Clontech, Palo Alto, CA, USA). 2.3.4 Functional analysis of PDS cDNA The PDS open reading frame was digested by EcoRI and BamHI and inserted into the corresponding sites of pUC18 (Stratagene, La Jolla, CA, USA) as an in-frame fusion to the lacZ gene, resulting in plasmid pUC-czPDS. The mutated PDS cDNA (with codon position 516 changed from a leucine codon to an arginine codon) was obtained by site-directed mutagenesis using the Quickchange mutagenesis kit (Stratagene). The mutation in the PDS cDNA (termed as czPDS-L516R) was verified by sequencing. E. coli strain JM109 was used as a host for functional expression experiments by co-transformation of pACCRT-EB that harbors the carotenoid biosynthesis genes for producing phytoene (Misawa et al., 1995) with plasmid pUC-czPDS or pUC-czPDS-L516R. Cells were grown in LB medium supplemented with 100 μg mL-1 ampicillin, 50 μg mL-1 chloramphenicol and 1 mM isopropylthiogalactose at 28 °C for 2 days. Pigments were extracted and analyzed according to Huang et al. (2006). E. coli cells were collected by centrifugation and then freeze-dried. Extraction was carried out with a mixture of dichloromethane and methanol (25:75, V/V) until the cell debris was almost 61 Chpater 2. Isolation and characterization of the phytoene desaturase gene from Chlorella zofingiensis colorless. The combined extracts were evaporated by nitrogen gas to dryness and dissolved in acetone for the subsequent high-performance liquid chromatography (HPLC) analysis. 2.3.5 RT-PCR assay Total RNA (1 µg) was extracted from C. zofingiensis cells illuminated with high light (120 µmol photons m-2 s-1) or treated with 50 mM glucose in the dark for 0 to 48 h. The RNA was reverse transcribed to cDNA with SuperScript III First-Strand Synthesis System for RT-PCR (Invitrogen, Carlsbad, CA) primed with oligo(dT) according to the manufacturer's instructions. The C. zofingiensis actin gene was used to normalize the level of RNA template used in the reaction (Huang et al., 2006). Amplification of the cDNA was done by conventional PCR [94 °C for 2 min followed by 26 cycles (for PDS gene) or 22 cycles (for actin gene) of 94 °C for 15 s, 56 °C for 15 s, 72 °C for 30 s]. PCR products were separated on 2% agarose gels and stained with ethidium bromide (EB) for photography (Biorad, Hercules, CA, USA). 2.3.6 Preparation of enzyme and substrate Plasmids pUC-czPDS and pUC-czPDS-L516R were respectively transformed into E. coli 109 for PDS expression. Transformed cells were grown in SOB medium containing 50 μg mL-1 ampicillin at 37 °C with vigorous shaking, and 1 mM IPTG was added when the optical density at 600 nm reached 0.5. Cells were harvested after a 5-h induction period, re-suspended in 0.1 × sodium phosphate buffer (pH 7.2, containing 1 mM DTT), and then passed through a French pressure cell (Spectronics Instruments, Rochester, NY, USA) at an internal pressure of 20 MPa. 10 μg mL-1 DNase was added to the broken cell extract and the mixture was incubated on ice for 15 min. Cell debris was removed from the suspension by centrifugation at 10,000 g and the resultant supernatant was adjusted to 1 mg mL-1 of crude protein and used as the source of PDS enzyme. Protein concentration was determined according to Bradford (1976). The substrate 62 Chpater 2. Isolation and characterization of the phytoene desaturase gene from Chlorella zofingiensis phytoene was extracted with acetone from E. coli JM109/pACCRT-EB freeze-dried cells according to procedures described by Breitenbach et al. (2001). 2.3.7 In vitro PDS assay The reaction mixture contained 1 mL of enzyme extract, 5 μL of the substrate phytoene (1 μg) in acetone, and 5 μL of decyl plastoquinone (10 mM solution in methanol, Sigma, St Louis, MO, USA) and 0.25 mg L-α-phosphatidylcholine (Sigma) in a sodium phosphate buffer suspension. The assays were carried out at 28 °C with vigorous shaking for various periods (0.5 to 12 h) to investigate the optimized incubation time for the enzymatic reaction. The reaction was terminated by the addition of 1 mL of methanol. To survey the herbicide resistance of phytoene desaturases, norflurazon (5 μL in methanol, Sigma) was added to the reaction mixture and incubated on ice for 15 min prior to mixing it with phytoene. Five final concentrations of norflurazon ranging from 0.05 μM to 0.8 μM for the unaltered PDS enzyme, and from 0.5 μM to 8 μM for the engineered PDS-L516R enzyme, were tested. The residual phytoene and enzymatically formed ζ-carotene were extracted from the incubation mixture with diethyl ether/petroleum ether (1:9, v/v), evaporated to dryness under a nitrogen stream and re-suspended in acetone for subsequent HPLC analysis. 2.3.8 Pigment analysis Extracted pigments were separated on a Waters Spherisorb® 5 μm ODS2 4.6 250 mm analytical column with a Waters HPLC system (Waters, Milford, MA, USA). The mobile phase consists of solvent A (acetonitrile/methanol/0.1 M Tris-HCl (pH 8.0), 84:2:14, by vol.) and solvent B (methanol/ethyl acetate, 68:32, v/v). Pigments were eluted at a flow rate of 1.2 mL min-1 with a linear gradient from 100% solvent A to 100% solvent B over a 15 min period, followed by 10 min of solvent B. The absorption spectra of the pigments were shown between 250 and 700 nm. Individual carotenoids were identified by their absorption spectra and their typical retention times compared to standard samples of pure 63 Chpater 2. Isolation and characterization of the phytoene desaturase gene from Chlorella zofingiensis carotenoids (Sigma). 2.4 Results and discussion 2.4.1 Cloning of C. zofingiensis PDS gene With the primers dF and dR (Table 2.1), a 140-bp fragment of the PDS gene was amplified (Figure 2.1, lane 1). Searches of the GenBank database using the BLAST program demonstrated that the nucleotide sequence of this fragment shared about 88% homology with that of C. reinhardtii and H. pluvialis. Based on this sequence information, two pairs of specific primers (F1, R1, F2, and R2 in Table 2.1) were designed for 5′ and 3′ RACE using the method described by Huang et al. (2006), which generated 2.1-kb fragment (Figure 2.1, lane 2). The sequence of the fragment was determined as a fusion of the 5′ and 3′ ends of a putative PDS cDNA. The coding region of the cDNA was amplified using the primers F3 and R3, which annealed to the start and stop codon regions, respectively. The PCR product (Figure 2.1, lane 3) was sequenced and contained a 1677-bp open reading frame encoding a deduced PDS with 558 amino acid residues (Figure 2.2A, GeneBank accession No. EF621405). Upstream of the translation start codon is a 117-bp 5′ untranslated region and between the stop codon and poly (A) tail is a 3′ untranslated region of 509 bp nucleotides. TGTAA, the sequence considered as a potential polyadenylation signal in green algae (Schmitt et al., 1992), however, is not found in the 3′ untranslated region of this PDS gene. The GC content of the PDS coding region is 50.8%, which is much lower than GC content of PDS genes from C. reinhardtii (63.7%) and H. pluvialis (58.6%). Protein sequence alignments showed that the PDS of C. zofingensis shared a high homology with that of other algae, cyanobacteria, and higher plants, and particularly with the green algae C. reinhardtii (74.6%) and H. pluvialis (70.0%) (Figure 2.3). No significant identity, however, is observed between the C. zofingensis PDS and its counterparts from bacteria and fungi, except in the N-terminal region which contains the dinucleotide binding motif (data not shown). Similar to H. pluvialis, the C. zofingensis PDS contains an N-terminal extension 64 Chpater 2. Isolation and characterization of the phytoene desaturase gene from Chlorella zofingiensis relative to the bacterial and fungal polypeptides. This extended sequence may serve as a transit peptide to direct the transport of PDS into plastids, since the PDS is nuclear-encoded but functions exclusively in the chloroplast (Grunewald et al., 2000). Furthermore, a transit peptide of 40 amino acids in the N-terminal extension of the PDS was predicted by using the ChloroP 1.1 software (http://www.cbs.dtu.dk/services/Chlorop). Table 2.1 Primer sets and PCR product characteristics Aim Oligonucleotide sequence 5′-3′ Partial PDS fragment Product size (kb) 0.14 dF GGCAAGGTNGCYGCNTGGAA dR GAGTGCTCCTTCCACTGCA 5′ and 3′ RACE 2.1 F1 AGGCCTGCACATCTTCTTTGGT R1 CCAGTCACCATCCTCATCCTTC F2 GATGAATGTATTTGCTGAACTGGGC R2 CCTTCCATGCGGCAACCTTGC PDS coding region* 1.7 F3 gcgaattccgATGCAACAGGCTCTAGGGCAG R3 ggggatccACTGTGCTGAGCTTGC 3′ PDS walking 0.8 F4 GTGGCAAGCTGGCTACTGAGG Ap1 GTAATACGACTCACTATAGGGC F5 CGTCACTGGAAGGTTGCACAC Ap2 ACTATAGGGCACGCGTGGT PDS gene 6.3 F6 GGCGCATAGGATTGACAAGCTT R6 GGGTGCCGCCGATCTGTGG PDS expression 0.29 F2 R7 GGCCAGTGCCTTAGCCATAGCG Site-directed mutation F8 (R8) CACCAAACAAAAGTACCgTGCATCCATGGAA GGTGCC (complement reverse) F: forward; R: reverse. R1, Ap1, R2 and Ap2 were also used for 5′ PDS walking. *EcoRI and BamHI sites (lower cases underlined) were added for cloning the gene into the corresponding cut sites of pUC 18 vector. 65 Chpater 2. Isolation and characterization of the phytoene desaturase gene from Chlorella zofingiensis To characterize the genomic PDS sequence, 5′ and 3′ genomic walking by PCR was performed and two fragments were obtained (Figure 2.1, lane 4 and 5). Sequencing results indicated that these two fragments contained the promoter and terminator regions of the PDS gene, respectively. Based on the sequence information of the isolated promoter and terminator fragments, the specific primers F6 and R6 were designed for the amplification of PDS gene and a 6.3-kb fragment was obtained (Figure 2.1 lane 6). Analysis of this amplified nucleotide sequence revealed that the product was the genomic sequence of PDS cDNA (GeneBank accession No. EF621406). The generated PDS gene is flanked by a 2-kb promoter region and a 560-bp terminator region, and its coding region is interrupted by six introns of 451, 217, 171, 111, 222 and 263 bp, respectively (Figure 2.2 B). All introns start with GT and end with CAG. Figure 2.1 PCR-based isolation of C. zofingiensis PDS gene. Specific fragments were amplified using genomic DNA (lane 1, 4, 5 and 6) or cDNA (lane 2 and 3) as template. Primers are listed in Table 1. Lane 1, dF+dR; lane 2, F1+R1 (first round) and F2+R2 (second round); lane 3, F3+R3; lane 4, R1+Ap1 (first round) and R2+Ap2 (second round); lane 5, F4+Ap1 (first round) and F5+Ap2 (second round); lane 6: F6+R6. M1, 100 bp ladder; M2, lambda DNA/StyI ladder (Fermentas). 2.4.2 Functional analysis of C. zofingiensis PDS cDNA in E. coli In algae the conversion of phytoene, the first C40-carotene in the carotenoid biosynthesis pathway, to ζ-carotene via phytofluene is catalyzed by 66 Chpater 2. Isolation and characterization of the phytoene desaturase gene from Chlorella zofingiensis Figure 2.2 Amino acid sequence alignment of C. zofingiensis PDS with its counterparts from A. thaliana (AAA20109), C. reinhardtii (AAT38476), D. salina (AAY26317), H. pluvialis (CAA60479) and Synechococcus sp. PCC 7942 (CAA39004). Amino acid residues which are either well or perfectly conserved in all sequences are indicated by (.) or (*) above the alignment, respectively. Primes are underlined with names. 67 Chpater 2. Isolation and characterization of the phytoene desaturase gene from Chlorella zofingiensis PDS through a two-step desaturation. To find out the enzymatic activity of the polypeptide encoded by the C. zofingiensis PDS cDNA, an in vivo functional expression analysis was performed. E. coli harboring pACCRT-EB produced the phytoene, which is colorless, while E. coli harboring both pACCRT-EB and pUC-czPDS exhibited a yellow color, indicating the formation of new pigments in the bacterium. HPLC analysis of extracts from the different E. coli transformants is shown in Figure 2.4. The E. coli carrying the plasmid pACCRT-EB accumulates phytoene (Figure 2.4A, peak 3). The transformants harboring both pACCRT-EB and pUC-czPDS mainly produced ζ-carotene (Figure 2.4B, peak 1); the level of residual phytoene was very low in these cells (Figure 2.4B, peak 3), suggesting that most of the substrate was converted to ζ-carotene. The intermediate product phytofluene was not observed (Figure 2.4B), possibly because it has a very short life time as a consequence of rapid desaturation to form the end product of the reaction. These results suggest that the C. zofingiensis PDS encodes a protein that rapidly converts phytoene to ζ-carotene. Figure 2.3 Schematic illustration of C. zofingiensis PDS cDNA (A) and its correspondent gene (B). The coding region of PDS cDNA is indicated by a black box; the promoter sequence is denoted by a gray box, the exons are showed by black boxes and the introns are line-indicated. 68 Chpater 2. Isolation and characterization of the phytoene desaturase gene from Chlorella zofingiensis 0.08 A 3 AU 0.06 0.04 0.02 0.00 18.00 0.006 19.00 B 20.00 21.00 22.00 23.00 24.00 Minutes 25.00 26.00 27.00 28.00 22.00 23.00 24.00 Minutes 25.00 26.00 27.00 28.00 22.00 23.00 24.00 Minutes 25.00 26.00 27.00 28.00 1 AU 0.004 0.002 3 0.000 18.00 19.00 C 20.00 21.00 1 AU 0.004 3 0.002 2 0.000 18.00 19.00 20.00 21.00 Figure 2.4 HPLC chromatogram of carotenoids extracted from E. coli cells harboring plasmid pACCRT-EB (A), pUC-czPDS and pACCRT-EB (B) or pUC-czPDS-L516R and pACCRT-EB (C). Absorbance was recorded at 305 nm. Peaks are identified as follows: 1 ζ-carotene, 2 phytofluene, 3 phytoene. 69 Chpater 2. Isolation and characterization of the phytoene desaturase gene from Chlorella zofingiensis 2.4.3 C. zofingiensis PDS gene is up-regulated by high light and glucose C. zofingiensis was found to accumulate ketocarotenoids in response to high light (Del Campo et al., 2004) or high concentrations of glucose (Ip et al., 2004; Ip & Chen, 2005). Whether the PDS gene is regulated by high light and glucose is unknown and investigated here. Photoautotrophically grown cells in exponential growth were either illuminated with high light (120 μmol photon m-2 s-1) or supplemented with glucose (50 mM) and maintained in the dark for 0 to 48 h. Basal expression of the PDS gene in control samples was observed (Figure 2.5A and B, lane 1). Basal expression of the PDS gene is required for the biosynthesis of carotenoids that serve as antenna pigments for light harvest during photosynthesis (Demmig-Adams & Adams, 1993). The level of PDS transcript was elevated upon exposure to high light, reaching its highest levels approximately 12 h following the high light exposure (Figure 2.5A). The increased steady-state expression of the PDS gene, in combination with expression of other genes of the carotenoid biosynthesis pathway, may contribute to the enhanced biosynthesis of ketocarotenoids. Interestingly, glucose also caused elevated PDS transcript accumulation (Figure 2.5B). The PDS expression was drastically up-regulated and reached its maximum upon 24-h glucose induction (Figure 2.5B, lane 3). Thereafter, the steady-state mRNA level began decreasing, finally to almost the basal level (Figure 2.5B, lane 4 and 5). The carotenogenic genes BKT and CHYb that are involved in astaxanthin biosynthesis were also found to be up-regulated by glucose (Huang et al., 2006; Li et al., 2008b, 2009). 70 Chpater 2. Isolation and characterization of the phytoene desaturase gene from Chlorella zofingiensis Figure 2.5 Analysis of the C. zofingiensis PDS expression under high light (120 μmol m-2 s-1) (A) or supplemented with 50 mM glucose (B) for 0 h (lane1), 12 h (lane 2), 24 h (lane 3), 36 h (lane 4) and 48 h (lane 5) using RT-PCR approach. Amplified product was separated on a 2% agarose gel, along with the control (actin) amplification (C). 2.4.4 PDS-L516R is resistant to the herbicide norflurazon Unlike the crtI-type phytoene desaturase from bacteria and fungi, the plant and algal PDS is the target of herbicides that inhibit the desaturation activity of PDS. However, PDS with specific amino acid changes become resistant to the herbicide norflurazon, as demonstrated in the cyanobacterium Synechococcus PCC 7942, (Chamovitz et al., 1991; Chamovitz et al., 1993), plants (Arias et al., 2006), and H. pluvialis (Steinbrenner & Sandmann, 2006). To determine whether the Chlorella PDS could be modified to become herbicide resistant, site-directed mutagenesis was used to generate an L516R change. Functional analysis of the altered protein was carried out in E. coli. As expected, ζ-carotene was produced in E. coli transformants carrying both pUC-czPDS-L516R and pACCRT-EB (Figure 2.4C). In addition, substantial amounts of the precursor phytoene and the intermediate phytofluene were present in transformants harboring the mutated PDS, but not in the tranformants with the unaltered protein (Figure 2.4C, peak 2 and 3). These data indicated that the mutation reduced the activity of phytoene desaturase, which is consistent with previous studies (Chamovitz et al., 1993; Michel et al., 2004). The time course of the formation of phytofluene and ζ-carotene from phytoene catalyzed by PDS was surveyed to optimize the incubation time for in 71 Chpater 2. Isolation and characterization of the phytoene desaturase gene from Chlorella zofingiensis vitro PDS assay. As indicated by Figure 2.6, both the unaltered PDS and PDS-L516R enzymes produced almost the highest amount of ζ-carotene after incubation of 6 h. Longer incubation time caused some degradation of the end product formed. These data suggested that a 6-h incubation time is sufficient for reliable quantitation of ζ-carotene and thus the PDS activity. PDS-L516R gave the lower amount of ζ-carotene and concurrent higher amount of the intermediate phytofluene as compared with the unaltered PDS (Figure 2.6), implying that this mutated enzyme had an attenuated desaturation activity, which is consistent with the results from functional analysis in E. coli (Figure 2.4). Figure 2.6 Time course of the formation of phytofluene (circle) and ζ-carotene (square) from phytoene catalyzed by the unaltered PDS (solid) and PDS-L516R (open). To explore the resistance of the mutated PDS to norflurazon, PDS-L516R was assayed in vitro. In vitro enzymatic activities of the unaltered PDS and PDS-L516R were measured by determining the conversion of phytoene to ζ-carotene after the addition of different concentrations of norflurazon. The results were expressed as units of activity relative to that of the control without the herbicide. A plot of the reciprocal of PDS enzymatic activities versus the concentration of the inhibitor norflurazon is shown in Figure 2.7. The 72 Chpater 2. Isolation and characterization of the phytoene desaturase gene from Chlorella zofingiensis concentrations for 50% inhibition (I50) of PDS desaturation activity were calculated to be 0.064 μM for the unaltered PDS and 2.237 μM for PDS-L516R. Compared with the unaltered PDS, the mutated one exhibited 35-fold higher resistance to the bleaching herbicide norflurazon. Figure 2.7 Plot of the reciprocal of in vitro PDS enzymatic activities versus concentrations of the bleaching herbicide norflurazon for the E. coli-expressing unaltered PDS (●) and PDS-L516R (■). RF stands for resistance factor. Of the enzymes involved in the formation of carotenoids, PDS is the primary target site of herbicides (Chamovitz et al., 1993; Michel et al., 2004). Inhibition of this enzyme causes phytoene accumulation at the expense of colored cyclic carotenoids. Thus in the presence of herbicides, there are not enough carotenoids formed to ensure efficient photoprotection of the photosynthetic apparatus. Chlorophylls therefore get degraded upon exposure of the cells to light, leading to the typical bleaching phenotype and subsequent deaths. In addition to causing herbicide resistance, the PDS mutations also reduce the enzymatic activity of phytoene desaturation to varying extents (Chamovitz et al., 1993; Michel et al., 2004). Since the herbicides do not compete for the binding of 73 Chpater 2. Isolation and characterization of the phytoene desaturase gene from Chlorella zofingiensis phytoene on PDS (Sandmann et al., 1989), it is speculated that the binding sites for the inhibitors and the substrate are either overlapping or in close proximity to each other (Chamovitz et al., 1993). The exact mechanism of herbicide inhibition, however, remains incompletely understood. Recently it was demonstrated that herbicides compete with cofactors of PDS for a binding site in the PDS polypeptide (Breitenbach et al., 2001). This binding site, in the PDS mutants, might be modified and thereby hinders the interaction with herbicides. The modification may also hinder the interaction of PDS with cofactors, resulting in a reduction in the efficiency of the phytoene desaturation. In conclusion, the PDS gene from C. zofingiensis encodes a polypeptide with high enzymatic activity in converting phytoene to ζ-carotene and was shown to be up-regulated by high light and glucose treatment. The modified PDS-L516R exhibited much higher resistance to the bleaching herbicide norflurazon than the unaltered PDS. The results suggested that the PDS-516R gene might be a useful tool for genetic engineering of carotenoid biosynthesis in C. zofingiensis because of its algal and endogenous origin and, more generally, may serve as a selectable marker for the introduction of endogenous DNA into algal genomes. 74 Chpater 3. Isolation and characterization of Chlorella zofingiensis mutants with enhanced biosynthesis of astaxanthin Chapter 3 Isolation and characterization of Chlorella zofingiensis mutants with enhanced biosynthesis of astaxanthin 3.1 Abstract The green alga Chlorella zofingiensis may serve as a new source of natural astaxanthin if its astaxanthin content gets increased. This chapter reported an effective system for generating and isolating astaxanthin-rich C. zofingiensis mutants. Mutations were generated by the employment of chemical mutagen MNNG (45 μg mL-1) or EMS (0.36 M) which gave an around 10% survival rate. Target mutants were isolated by screening the treated cells with 0.5 μM norflurazon. Over two hundreds mutants were obtained, from which five were selected for further characterization. No significant differences in cell growth and pigment profile were found between the mutants and wild type (WT) cells when the culture medium contained no herbicide. In contrast to WT cells which were bleached by 0.25 μM norflurazon, all the mutants grew well and synthesized astaxanthin even when the culture medium contained up to 1 μM norflurazon. Furthermore, mutants accumulated up to 54% more astaxanthin than WT. Coordinately, higher transcript levels of carotenoid ketolase (BKT) and carotenoid hydroxylase (CHYb) genes were found in the mutant cells. Interestingly two of the five mutants also accumulated higher amounts of total carotenoids (TC). These results indicates that C. zofingiensis and possibly other green algae can be genetically modified by mutagenesis followed by specific herbicide screening for enhanced production of carotenoids including the high-value ketocarotenoid astaxanthin. 75 Chpater 3. Isolation and characterization of Chlorella zofingiensis mutants with enhanced biosynthesis of astaxanthin 3.2 Introduction The major constraint of C. zofingiensis as commercial source of natural astaxanthin is its low cellular content of astaxanthin. One strategy to overcome this problem is to genetically modify the key enzymes involved in the carotenoid biosynthesis. Mutagenesis has been found to be an effective approach to enhance the biosynthesis of carotenoids including astaxanthin in various algal species, especially in H. pluvialis (Tjahjono et al., 1994; Zhang & Lee, 1997; Chen et al., 2003; Ishikawa et al., 2004; Hu et al., 2008a; Sandesh Kamath et al., 2008). Commonly chemical mutagen (e.g., EMS or MNNG) or UV is used to generate mutants from which target mutants (e.g., cells with higher contents of certain carotenoids) are selected by using specific inhibitors to carotenogenic enzymes. While a number of astaxanthin hyper-producing mutants of H. pluvialis have been obtained, no mutants of other astaxanthin-producing green algae are available. In addition, there is little information on the mechanisms of metabolic processes of such mutants. This chapter described for the first time the generation and selection of C. zofingiensis mutants with over-production of astaxanthin through chemical mutagenesis followed by herbicide screening. Furthermore, the expression of carotenogenic genes including phytoene desaturase (PDS), carotenoid ketolase (BKT) and carotenoid hydroxylase (CHYb) were investigated among WT and mutants. 3.3 Methods and materials 3.3.1 Algal strain and culture conditions The maintenance and inoculation of C. zofingiensis were described in 2.3.1. 76 Chpater 3. Isolation and characterization of Chlorella zofingiensis mutants with enhanced biosynthesis of astaxanthin 3.3.2 Mutagenesis Cells from logarithmic suspension culture were harvested, washed twice with distilled water and re-suspended in 0.1 M phosphate buffer (pH 7.0) at a concentration of 108 cells mL-1 for mutagenesis. The cells mentioned above were treated with ethyl methanesulphonate (EMS, Sigma) in the concentration range of 0 to 0.48 M for 30 min or with N-methyl-N′-nitro-N-nitrosoguanidine (MNNG, Sigma) at 0 to 60 μg L-1 concentration for 60 min. 3.3.4 Isolation of Chlorella mutants After the treatment of chemical mutagens, equal volume of filter-sterilized sodium thiosulfate (5%) was added to terminate the mutagenesis. All the treated cells were washed twice with distilled water, re-suspended in Kuhl medium and allowed to grow for 24 h (Hirschberg et al., 1987). For selection, the mutagenized cells were incubated on Kuhl agar plates containing 0.5 μM norflurazon at 25 °C under continuous illumination of 25 µmol photon m-2 s-1. The colonies showing up on the selection plates were picked up based on the colony characteristics and color and transferred individually into Kuhl liquid medium containing 0.5 μM norflurazon for cultivation. 3.3.5 Cell dry weight determination The cultures of WT and mutants were centrifuged at 3,800 g for 5 min. The pellet was washed three times with distilled water and filtered through a pre-dried Whatman GF/C filter paper (1.2 μm pore size). The algal cells on the filter paper discs were dried at 70 °C in a vacuum oven until constant weight and were cooled down to room temperature in a desiccator before weighting. 77 Chpater 3. Isolation and characterization of Chlorella zofingiensis mutants with enhanced biosynthesis of astaxanthin 3.3.6 Astaxanthin induction The cultures of WT and mutants were first grown in Kuhl medium for 4 days. To induce astaxanthin accumulation, the cultures mentioned above were inoculated into the fresh medium containing 30 g L-1 glucose and cultured in the absence of light. 3.3.7 Extraction and analysis of pigments Cell samples were collected by centrifugation and then freeze-dried on a DW3 freeze-drier (Heto Dry Winner, Denmark). Extraction was carried out with acetone and liquid nitrogen until the cell debris was almost colorless. The extracts were filtered through a 0.22 μm Millipore organic membrane. HPLC analysis of the pigments was described in 2.3.7. 3.3.8 RNA isolation and RT-PCR assay RNA isolated and reverse transcription were described in 2.3.5. PCR amplification was carried out using specific primers of PDS, BKT and CHYb (Table 3.1). C. zofingiensis actin (ACT) primers (Table 3.1) were used to demonstrate equal amounts of templates and loading. The GenBank accession numbers for PDS, BKT and CHYb were EF621406, AY772713 and EU016205, respectively. Amplification was done by conventional PCR [94 °C for 2 m followed by 24 cycles (for PDS, BKT and ACT genes) or 26 cycles (for CHYb gene) of 94 °C for 15 s, 58 °C for 20 s, 72 °C for 30 s]. 78 Chpater 3. Isolation and characterization of Chlorella zofingiensis mutants with enhanced biosynthesis of astaxanthin Table 3.1 Primer sets for gene expression by RT-PCR Primer (5′-3′) Gene PDS Forward Reverse BKT Forward Reverse CHYb Forward Reverse ACT Forward Reverse GATGAATGTATTTGCTGAACTGGGC GGCCAGTGCCTTAGCCATAGCG GTGCTCAAAGTGGGGTGGTATG CCATTTCCCACATATTGCACCT GCCAGCCATGAAACGTGTG GTTCCTTCCAGTTATGTACACA TGCCGAGCGTGAAATTGTGAG CGTGAATGCCAGCAGCCTCCA 3.4 Results 3.4.1 Carotenoid biosynthesis blocked by norflurazon C. zofingiensis cells exhibited green phenotype under standard growth conditions. Exposed to high light irradiation or induced by glucose, ketocarotenoids mainly astaxanthin and canthaxanthin accumulated within cells, rendering the cells an orange-red color (Orosa et al., 2000, 2001; Del Campo et al., 2004; Ip et al., 2004; Ip & Chen, 2005; Li et al., 2009). Norflurazon can specifically bind to PDS and block phytoene desaturation, giving rise to phytoene accumulation at an expense of colored cyclic carotenoids (Chamovitz et al., 1993). Without sufficient carotenoids formed to ensure efficient photoprotection of photosynthetic apparatus, chlorophylls get degraded upon exposure of cells to light, leading to the typical bleaching phenotype and subsequent death of cells. Various concentrations of norflurazon were employed to examine its effect on carotenoid formation in C. zofingiensis cells. As shown in Figure 3.1, TC content 79 Chpater 3. Isolation and characterization of Chlorella zofingiensis mutants with enhanced biosynthesis of astaxanthin in the algal cells exhibited high sensitivity to noflurazon. In the presence of 0.1 μM norflurazon, TC content reduced from 1.685 mg g-1 (control value of culture with no herbicide) to 0.336 mg g-1. Higher concentrations of norflurazon (0.25 μM, 0.5 μM, and 1 μM) resulted in further lower TC contents, and colorless phytoene was found to accumulate in these norflurazon-treated cells (data not shown). Figure 3.1 Effect of norflurazon on TC accumulation in C. zofingiensis cells. Cell samples were harvested after 4-day cultivation under standard growth condition supplemented with various concentrations of norflurazon. 3.4.2 Isolation of Chlorella mutants resistant to norflurazon Appropriate levels of MNNG and EMS were determined by treating C. zofingiensis cells in various concentrations. The survival rate of C. zofingiensis treated with MNNG decreased rapidly and reached 11.4% when the concentration of MNNG increased to 45 μg mL-1 (Figure 3.2A). EMS also reduced the survival rate in a concentration-dependent manner and gave 9.6% survival rate at 0.36 M (Figure 3.2B). The algal cells treated with 45 μg mL-1 MNNG for 1 h or 0.36 M EMS 80 Chpater 3. Isolation and characterization of Chlorella zofingiensis mutants with enhanced biosynthesis of astaxanthin Figure 3.2 Effect of MNNG (A) and EMS (B) treatments on the survival rate of C. zofingiensis cells. The survival rate of each treatment was counted by comparing with control cells (100% survival). for 30 min were selected further by spreading onto solid media supplemented with 0.5 μM norflurazon. Green colonies were observed after 2 to 3 weeks of incubation. More than 100 colonies for each treatment were obtained. Mutants that were found to show apparent deeper orange color were selected for further investigation. The selected colonies were transferred into liquid medium containing 0.5 μM norflurazon and allowed to grow under standard growth conditions. To eliminate negative effects of herbicide on algal cells, the survived colonies were inoculated into norflurazon free medium twice for further studies. 81 Chpater 3. Isolation and characterization of Chlorella zofingiensis mutants with enhanced biosynthesis of astaxanthin 3.4.3 Growth and astaxanthin accumulation of mutants and WT Under standard growth conditions (without norflurazon), all mutants showed a similar growth pattern to WT and no significant difference of cell biomass was observed among WT and mutants (data not shown). Norflurazon greatly limited the cell growth of WT in a concentration dependent manner (Figure 3.3A). In contrast, mutants exhibited strong resistance to the herbicide. As shown in Figure 3.3A, 0.25 μM norflurazon drastically reduced the cell biomass of WT from 4.64 g L-1 (control value of culture with no herbicide) to 2.82 g L-1 but only slightly affected the cell biomass of mutants. Even in the presence of 1 μM norfluraon, the cell biomass and chlorophyll content of the mutants was only moderately reduced to 3.54 g L-1 and 2.25 mg g-1 respectively (for E17, Figure 3.3A and Table 3.2). WT cells were totally bleached in the presence of 1 μM norflurazon, correlated with the drastic decrease of chlorophyll content (Table 3.2). Table 3.2 Chlorophyll content of C. zofingiensis WT and mutants after 4-day cultivation with or without norflurazon Chlorophyll content (mg g-1) Strains Norflurazon free 1 μM norflurazon WT 6.51 ± 0.46 0.41 ± 0.04 M7 5.89 ± 0.53 2.32 ± 0.19 M87 6.24 ± 0.39 2.47 ± 0.28 E5 5.66 ± 0.52 2.11 ± 0.23 E17 6.11 ± 0.33 2.25 ± 0.17 E65 6.03 ± 0.41 2.20 ± 0.35 Norflurazon also exerted a negative effect on astaxanthin formation in algal cells of both WT and mutants. However, astaxanthin accumulation in WT 82 Chpater 3. Isolation and characterization of Chlorella zofingiensis mutants with enhanced biosynthesis of astaxanthin cells was much more sensitive to norflurazon than that in mutants. As illustrated by Figure 3.3B, in the presence of 0.1 μM norflurazon, astaxanthin content in WT cells was greatly reduced from 0.235 mg g-1 to 0.014 mg g-1, while in mutants the astaxanthin contents were only slightly reduced (from 0.273 mg g-1 to 0.212 mg g-1). Inhibitory concentration that inhibits astaxanthin formation by 50% (I50) was calibrated by using Dixon plot as [norflurazon (μM)] versus [astaxanthin content (mg g-1)]-1. The norflurazon-resistant mutants obtained in this study exhibited 21to 26- fold higher resistance than WT (Table 3.3). Figure 3.3 Effect of norflurazon concentrations on cell biomass (A) and astaxanthin contents (B) of mutants. Cell samples were harvested after 4-day cultivation under standard growth condition. 83 Chpater 3. Isolation and characterization of Chlorella zofingiensis mutants with enhanced biosynthesis of astaxanthin Table 3.3 Half-maximum inhibitory concentration (I50) of norflurazon on astaxanthin formation in C. zofingiensis WT and mutants Strains I50 (μM) WT 0.021 M7 0.485 M87 0.532 E5 0.448 E17 0.544 E65 0.525 3.4.4 Accumulation of TC and astaxanthin induced by glucose The algal cells of WT and mutants were first allowed to grow for 4 days under standard growth conditions, followed by glucose induction for 5 days. The induced cells were harvested and freeze-dried for pigment extraction and HPLC analysis. M7, M87 and E5 produced higher amounts of astaxanthin, while the TC contents of these three mutants were comparable to the WT (Figure 3.4A). The astaxanthin contents in M7, M87 and E5 were 0.661 mg g-1, 0.65 mg g-1 and 0.621 mg g-1, 48.5%, 46.1% and 39.6% greater than that in the WT (0.445 mg g-1), respectively. In contrast, E17 and E65 could accumulate greater amounts of both astaxanthin and TC (Figure 3.4A). Among the mutants investigated in the present study, E17 had the ability to accumulate highest amounts of TC and astaxanthin, which were 2.277 mg g-1and 0.685 mg g-1, 30.1% and 53.9% higher than WT, respectively. Correlated with the higher astaxanthin content, the mutant cells exhibited deeper yellow color than the WT (Figure 3.4B). 84 Chpater 3. Isolation and characterization of Chlorella zofingiensis mutants with enhanced biosynthesis of astaxanthin Figure 3.4 Comparison of WT and mutants cultured with 30 g L-1 glucose in the dark. (A) TC and astaxanthin accumulated in WT and mutants. (B) Algal cells of WT and mutants. Cell samples were harvested after glucose induction for 5 days. Gray and dark columns indicate TC and astaxanthin respectively. Data marked with the same letters of each total carotenoid content and astaxanthin are not significantly different (P>0.05). 85 Chpater 3. Isolation and characterization of Chlorella zofingiensis mutants with enhanced biosynthesis of astaxanthin 3.4.5 Expression analysis of carotenogenic genes Three genes including PDS, BKT and CHYb that are associated with general carotenogenesis and specific astaxanthin biosynthesis were selected for expression analysis. The transcript levels of these genes in WT and mutants were quantified by RT-PCR and compared. Algal cells induced with glucose for 48 h were employed. All mutants exhibited higher transcription levels of BKT and CHYb than WT while the increasing extent differed (Figure 3.5), which was well consistent with the greater amount of astaxanthin produced by mutants. No significant difference in PDS transcript, however, was observed between WT and mutants (M7, M87 and E5) (Figure 3.5). Even in E17 and E65 that produced notable higher amounts of TC, the PDS expression was merely comparable to WT. Figure 3.5 Expresson of carotenoid biosynthetic genes in cells of WT and mutants. Cell samples were induced for 2 days with glucose in the dark. The PCR amplified products were separated on a 2% agarose gel, along with the control (ACT) amplification. 3.5 Discussion In this chapter, chemical mutagens MNNG and EMS were employed to mutate C. zofingiensis cells for enhanced astaxanthin production. Various concentrations of the mutagens were surveyed to obtain satisfactory survival rates 86 Chpater 3. Isolation and characterization of Chlorella zofingiensis mutants with enhanced biosynthesis of astaxanthin of the algal cells. Cells treated with 45 μg mL-1 MNNG or 0.36 M EMS giving approximately 10 % survival rate were found practicable to obtain sufficient mutants from which astaxanthin over-production mutants were selected by screening with 0.5 μM norflurazon. Herbicides are a group of diverse chemical compounds with the ability of disturbing basic metabolic processes essential for algal cells. They have been used effectively to screen mutants with desired properties, including but not restricted to herbicide resistance (Hirschberg et al., 1987; Linden et al., 1990; Tjahjono et al., 1994), enhanced accumulation of carotenoids (Tripathi et al., 2001; Chen et al., 2003; Sandesh Kamath et al., 2008). Astaxanthin-hyperproducing mutants of H. pluvilias have been isolated by using chemical mutagenesis followed by screening with inhibitors of the carotenoid biosynthesis (e.g., diphenylamine, compactin or nicotine) (Tripathi et al., 2001; Chen et al., 2003). In this chapter, over 200 norflurazon-resistant mutants of C. zofingiensis were obtained, of which 5 mutants were further investigated. These mutants accumulated higher amounts of astaxanthin than WT under high light irradiation (Figure 3.4). In addition to astaxanthin, the EMS-treated mutants E17 and E65 also produced a greater amount of TC (Figure 3.4A). The enhanced astaxanthin production in the mutants might be related to the increased expression of both BKT and CHYb genes, based on the RT-PCR analysis results (Figure 3.5), since astaxanthin formation was previously shown to be related to the transcript levels of carotenogenic genes (Li et al., 2008, 2009). Although the EMS-treated Synechococcus sp. mutants and MNNG-induced H. pluvialis mutants were suggested to be related to a single-gene mutation (Linden et al., 1990; Hu et al., 2008a), it remains unknown at this point whether the five C. zofingiensis mutants obtained in the present study were the result of a single- or multi-gene mutation. A point mutation might occur in the PDS gene of the C. zofingiensis mutants, resulting in resistance to the bleaching herbicide norflurazon (Chamovitz et al., 1993). In addition, it would be likely that a key regulatory gene involved in the biosynthesis of carotenoids also got mutated, 87 Chpater 3. Isolation and characterization of Chlorella zofingiensis mutants with enhanced biosynthesis of astaxanthin therefore redirecting the carotenoid flux to astaxanthin and promoting astaxanthin accumulation in the C. zofingiensis mutants. The underlying mechanism responsible for the enhanced accumulation of astaxanthin in mutants remains to be elucidated and further studies will be described in chapter 4. Astaxanthin has been widely used in aquaculture for pigmentation of many marine animals such as salmon and trout. Around 100, 000 kg of astaxanthin per year is demanded, with a price estimated at US$200 million (Lorenz & Cysewski, 2000). Essentially, today’s commercial astaxanthin for aquaculture is almost produced by chemical synthesis, but consumer’s growing need using natural ingredients in all forms of food nutrients provides a good chance for natural astaxanthin production by green microalgae. H. pluvialis is the most promising source of astaxanthin for aquaculture in that it has the highest cellular content of astaxanthin and no further purification of the pigment is required (Ausich, 1997). However, its slow growth rate, low cell yield and susceptibility to contamination hinder its commercial application (Olaizola, 2000; Hata et al., 2001; Ip et al., 2004). Furthermore, light is required for enhancing astaxanthin production by H. pluvialis cells (Orosa et al., 2000; Hata et al., 2001), adding cost to commercialization of algal products. Recently C. zofingienesis has drawn much attention and been proposed as an alternative promising producer of astaxanthin due to its fast growth and ability to accumulate astaxanthin in the dark, suggesting that it might be well developed for producing astaxanthin in industrial fermentation systems (Orosa et al., 2000; Ip et al., 2004; Ip & Chen, 2005). Very high biomass (53 g L-1) of C. zofingienesis has been achieved by using a fed-batch fermentation strategy (Sun et al., 2008). Thus, the low cellular astaxanthin content of C. zofingiensis is the major limitation to its commercial use as natural astaxanthin. The E17 has the ability to accumulate approximately 54% more cellular astaxanthin than the WT. The mutants described in this study may be further modified for enhanced production of astaxanthin by a similar approach used in this study but selecting by using other herbicides (e.g., nicotine or diphenylamine) that target different carotenogenic enzymes. In addition, the 88 Chpater 3. Isolation and characterization of Chlorella zofingiensis mutants with enhanced biosynthesis of astaxanthin mutants also provide important materials for the structure-function relationship of the membrane PDS enzyme. 89 investigation of Chpater 4. Molecular characterization of the Chlorella zofingiensis mutant E17 Chapter 4 Molecular characterization of the Chlorella zofingiensis mutant E17 4.1 Abstract A stable Chlorella zofingiensis mutant (E17) resistant to the herbicide norflurazon was characterized with respect to growth, astaxanthin biosynthesis, and phytoene desaturation. The mutant E17 could grow well and produced normal levels of colored carotenoids in the presence of 0.25 μM norflurazon, in which the growth of WT cells was greatly limited due to inhibited carotenoid formation. Induced by high-light irradiation or glucose, E17 produced 44% or 36% more astaxanthin than WT when cultured in media without norflurazon. A point mutation (C to T) was revealed to occur in the PDS gene of E17, leading to an amino acid change (L516F) in its coding region. The mutated PDS exhibited 31-fold resistance to norflurazon when compared to WT as determined by an in vitro assay. Surprisingly, the mutated PDS exhibited higher efficiency in converting phytoene to ζ-carotene. No difference in PDS transcripts was found between E17 and WT cells cultured either in normal or induced conditions. In contrast, higher transcript levels of BKT and CHYb were found in E17 cells. These results suggest that a point mutation in Chlorella PDS gene results in an enzyme with lower affinity to norflurazon but higher efficiency in converting phytoene to ζ-carotene. As a consequence, E17 is resistant against norflurazon and synthesizes higher amounts of carotenoids including astaxanthin. 90 Chpater 4. Molecular characterization of the Chlorella zofingiensis mutant E17 4.2 Introduction A number of norflurazon-resistant mutants of C. zofingiensis by chemical mutagenesis were generated in chapter 3. One of them, named as E17, is a stable mutant that had a higher astaxanthin content than the WT. Norflurazon inhibits the activity of plant-type phytoene desaturase (PDS), a key enzyme involved in catalyzing the rate-limiting step of carotenoid biosynthesis (Chamovitz et al., 1993). Mutants resistant to norflurazon may result from the increased amount of PDS gene product or from structural change of PDS that lowers its affinity to the herbicide (Chamovitz et al., 1991, 1993; Michel et al., 2004). In this chapter, the characterization of E17 was carried out, with respect to its growth, carotenogenesis and PDS activity. This mutated PDS is the first example for a point mutation in PDS which not only makes the alga resistant to the herbicide norflurazon, but also enhances the biosynthesis of total carotenoids (TC) including astaxanthin. 4.3 Methods and materials 4.3.1 Algal strain and culture conditions The maintenance and inoculation of C. zofingiensis were described in 2.3.1. For herbicide treatment, the cell cultures were grown in Kuhl medium for 4 days and then inoculated into the same medium containing various concentrations of norflurazon (Sigma), at an inoculum of 10% (by volume, average cell concentration of 0.5 g L-1). For induction of astaxanthin biosynthesis, the 4-day cultures mentioned above were exposed to continuous illumination of 250 µmol photon m-2 s-1 or inoculated into fresh Kuhl medium containing 30 g L-1 glucose and maintained in the dark. 91 Chpater 4. Molecular characterization of the Chlorella zofingiensis mutant E17 4.3.2 Cell dry weight determination Cell dry weight determination was described in 3.3.5. 4.3.3 Extraction and analysis of pigments The extraction and pigment analysis were described in 3.3.7. 4.3.4 Chlorophyll fluorescence measurement Cells were harvested by centrifugation and re-suspended in fresh Kuhl medium. Chlorophyll fluorescence was measured using a Multi-Mode Chlorophyll Fluorometer (Opti-Sciences, Hudson, USA) at room temperature. Non-photochemical quenching (NPQ) was calculated using (Fm-Fm′)/Fm′, where Fm is the maximum fluorescence in the dark-adapted state and Fm′ is the maximum fluorescence in the light-adapted state. 4.3.5 RNA isolation and RT-PCR assay RNA isolation and RT-PCR assay were described in 3.3.8. 4.3.6 PDS expression in E. coli The PDS cDNA from E17 was amplified, sequenced and inserted into pUC18 (Stratagene) as an in-frame fusion to the lacZ gene, resulting in plasmid pUC-czPDS-L516F (a leucine codon at position 516 changed to a Phenylalanine codon). This plasmid and pUC-czPDS constructed in chapter 2 were introduced into E. coli JM109 for PDS enzyme expression. The E. coli cells were grown in 92 Chpater 4. Molecular characterization of the Chlorella zofingiensis mutant E17 SOB medium containing 50 μg mL-1 ampicillin at 37 °C with vigorous shaking, and 1 mM IPTG was added when the optical density at 600 nm reached 0.5. After a 5-h induction period the cells were harvested for preparation of crude PDS proteins. 4.3.7 Enzyme and substrate preparation and in vitro PDS assay The preparation of enzyme and substrate and the in vitro PDS assay were described in 2.3.6 and 2.3.7, respectively. 4.4 Results 4.4.1 The growth and carotenogensis of E17 Chapter 3 described the treatment of C. zofingiensis cells with chemical mutagen EMS followed by selection with the bleaching herbicide norflurazon. A number of mutants resistant to norflurazon were obtained and analyzed for their astaxanthin production. E17, a stable mutant, was found to produce much more astaxanthin. E17 showed no loss of resistance to norflurazon after over 100 times of subculture without norflurazon selection (data not shown). E17 could grow well and produce nearly normal levels of total carotenoids in the presence of 0.25 µM norflurazon, in which the growth of wild type (WT) cells was greatly limited due to total blocking of carotenoid formation. Norflurazon suppressed carotenogenesis leading to a decreased formatio of TC including astaxanthin in both WT and E17 cells (Figure 4.1). WT cells showed high sensitivity to norflurazon as indicated by a decreased TC content (from 1.69 mg g-1 to 0.83 mg g-1) in the present of 0.05 μM norflurazon. Higher concentrations of norflurazon resulted in much lower amounts of TC (Figure 4.1). In addition, the formation of astaxanthin in WT almost ceased when the cultures were treated with up to 0.25 93 Chpater 4. Molecular characterization of the Chlorella zofingiensis mutant E17 μM norflurazon. In contrast, E17 synthesized significant amounts of TC and astaxanthin even at 0.5 μM norflurazon, a lethal concentration for the WT. Values of half-maximum inhibitory concentration (I50) of norflurazon on cell biomass, TC and astaxanthin formation in WT and E17 was shown in Table 4.1. E17 exhibited 7.1, 22.7 and 25.9 -fold higher resistance to norflurazon than WT, with respect to cell biomass, TC accumulation and astaxanthin formation respectively. Figure 4.1 Effect of norflurazon concentration on TC and astaxanthin contents of WT and E17. (□) WT TC; (■) WT astaxanthin; (○) E17 TC; (●) E17 astaxanthin. Cells were harvested from 4-day culture under standard growth conditions. Table 4.1 Half-maximum inhibitory concentrations (I50) of norflurazon on cell biomass, TC and astaxanthin formation of WT and E17. I50 was calibrated by using Dixon plot as [norflurazon (μM)] versus [biomass (g L-1)], [TC content (mg g-1)] or [astaxanthin content (mg g-1)]. I50 (μM) Strain Cell biomass TC WT 0.395 0.034 0.021 E17 2.802 0.702 0.544 94 Astaxanthin Chpater 4. Molecular characterization of the Chlorella zofingiensis mutant E17 4.4.2 Enhanced production of TC and astaxanthin by E17 under high light stress or glucose induction High light irradiation was reported to trigger ketocarotenogensis in C. zofingiensis cells (Orosa et al., 2000, 2001; Del Campo et al., 2004; Li et al., 2009). Here a comparative study of WT and E17, in terms of TC and astaxanthin contents under high light irradiation, was conducted. Consistent with previous reports, C. zofingiensis WT and E17 synthesized much higher amounts of TC and astaxanthin under high light than normal light (Figure 4.2A). E17 produced apparently greater amounts of both TC and astaxanthin than WT during the whole illumination period (Figure 4.2A). The carotenoid contents of WT and E17 cells exposed to high light for 24 h are shown in Table 4.2. Lutein was the major carotenoid, followed by zeaxanthin and astaxanthin. Significant higher amounts of β-carotene, lutein, zeaxanthin, as well as the ketocarotenoids adonixanthin, canthaxanthin and astaxanthin were found to accumulate in E17 cells. The TC and astaxanthin contents (2.96 mg g-1 and 0.38 mg g-1) of E17 were 27.9 % and 43.7% higher than that of WT respectively. The zeaxanthin content and the zeaxanthin/(zeaxanthin + antheraxanthin + violaxanthin) ratio, two factors related to the degree of NPQ, were both increased in E17 when compared with WT (Table 4.2). In accordance with the higher zeaxanthin content, E17 had a greater NPQ value (Figure 4.3). Both WT and E17 reached their maxima at 105 s and the NPQ value of E17 was 33.3% higher than that of WT. In addition to light, glucose is another inducer of astaxanthin biosynthesis in C. zofingiensis cells (Ip et al., 2004). With glucose as the sole carbon and energy source, C. zofingiensis grew fast and synthesized astaxanthin (Ip & Chen, 2005; Sun et al., 2008). The time course of TC and astaxanthin accumulation in heterotrophic WT and E17 cells cultured in Kuhl medium with 30 g L-1 is shown in Figure 4.2B. Compared with the non-induced cells on day 0, the TC and astaxanthin contents of WT were slightly decreased on day 2. Thereafter both TC 95 Chpater 4. Molecular characterization of the Chlorella zofingiensis mutant E17 and astaxanthin increased and the astaxanthin content reached its maximum in 14 days. E17 exhibited the same induction pattern of TC and astaxanthin as WT. However, E17 produced apparently greater amounts of carotenoids, for example on day 6 the TC and astaxanthin contents were 2.50 mg g-1and 0.85 mg g-1, 18% and 36% higher than that of WT respectively. Even on day 10 the astaxanthin content of WT was slightly lower than that of E17 on day 6. It is worth noting that after day 6 the difference in TC and astaxanthin contents between WT and E17 became smaller. In correlation with the earlier enhanced astaxanthin accumulation, E17 exhibited an earlier change from green to orange (data not shown). Figure 4.2 Accumulation of TC and astaxanthin in WT and E17 cells under continuous illumination of high light (A) or under induction of 30 g L-1 glucose in the dark (B). (□) WT TC; (■) WT astaxanthin; (○) E17 TC; (●) E17 astaxanthin. 96 Chpater 4. Molecular characterization of the Chlorella zofingiensis mutant E17 Table 4.2 Pigment contents of green cells of WT and E17 exposed to high light for 24 h a Carotenoid composition (μg g-1) TC (mg g-1) Chlorophyll (mg g-1) Zea / (vio + Neo Vio Ant Ast Ado Lut Zea Can β-Car Vio + ant + ant + zea) zea WT 2.31 ± 0.09 6.23 ± 0.21 64 ± 2.1 38 ± 1.3 67 ± 3.1 261 ± 11 219 ± 8 1132 ± 48 300 ± 11 128 ± 4 101 ± 5 405 0.74 E17 2.96 ± 0.12 6.05 ± 0.27 75 ± 3.4 32 ± 1.5 64 ± 1.8 375 ± 15 297 ± 13 1397 ± 55 414 ± 17 160 ± 6 121 ± 3 510 0.81 a Data were expressed as the mean of three independent experiments. Neo, neoxanthin; Vio, violaxanthin; Ant, antheraxanthin; Ast, astaxanthin; Ado, adonixanthin; Lut, lutein; Zea, zeaxanthin; Can, canthaxanthin; β-Car, β-carotene 97 Chpater 4. Molecular characterization of the Chlorella zofingiensis mutant E17 Figure 4.3 Kinetics of NPQ of dark-adapted WT (■) and E17 (●) measured in 24-h high light treated cells. 4.4.3 Characterization of E17 PDS Norflurazon can be coped with by increased amounts of the target enzyme or by a mutation that affects the binding properties. The sequencing of the PDS gene from E17 revealed a single point mutation (C to T), which resulted in an amino acid change from leucine (L) to phenylalanine (F) at codon 516 (from CTC to TTC). This L residue is located in a conserved motif of the PDS polypeptides. Another substitution of this L by arginine (R) was earlier revealed by us to confer herbicide resistance on Chlorella PDS (Huang et al., 2008). To verify whether PDS-L516F is related to norflurazon resistance, the in vitro assays of the Chlorella PDS and PDS-L516F were carried out. In vitro desaturation activities of the unaltered PDS and PDS-L516F were measured by determining the conversion of phytoene to ζ-carotene in the presence of various concentrations of norflurazon. The results were expressed as units of activity relative to that of the control without the herbicide. A Dixon plot of the reciprocal of PDS 98 Chpater 4. Molecular characterization of the Chlorella zofingiensis mutant E17 desaturation activity versus the concentration of norflurazon is shown in Figure 4.4. The concentrations for 50% inhibition (I50) of PDS desaturation activity were calculated to be 0.072 μM for WT PDS and 2.242 μM for PDS-L516F, respectively. Compared with the WT PDS, the mutated one showed 31-fold greater resistance to the inhibitor norflurazon. Surprisingly, PDS-L516F exhibited higher activity in converting phytoene to ζ-carotene than its unaltered counterpart as well as the PDS-L516R that was shown to have attenuated desaturation activity (Chamovitz et al., 1993; Steinbrenner & Sandmann, 2006; Huang et al., 2008). As shown in Table 4.3, the specific activity of PDS-L516F was 0.0315 μg ζ-carotene formed (mg protein)-1 h-1, about 29% and 50% higher than that of the unaltered PDS and PDS-L516R, respectively. Figure 4.5 Plot of the reciprocal of in vitro phytoene desaturase activities versus concentrations of norflurazon for the E. coli-based recombinant unaltered PDS (●) and PDS-L516F (■). 99 Chpater 4. Molecular characterization of the Chlorella zofingiensis mutant E17 Table 4.3 In vitro phytoene desaturation activity of E. coli-epxressing PDS proteins. Crude enzymes Specific activity [μg ζ-carotene formed (mg protein)-1 h-1] PDS 0.0245±0.0015 PDS-L516R 0.0193±0.0012 PDS-L516F 0.0315±0.0021 4.4.4 Transcription analysis of PDS, BKT and CHYb genes It was earlier reported that the enhanced biosynthesis of carotenoids including astaxanthin in C. zofingiensis by high light or glucose induction was correlated to the increased transcript levels of carotenogenic genes (Li et al., 2008b; Li et al., 2009). To further investigate whether the enhanced production of TC and astaxanthin by E17 was attributed to the up-regulation of PDS and/or other carotenogenic genes, the quantification of the transcript levels of PDS together with BKT and CHYb, two enzymes directly involved in astaxanthin formation from β-carotene, was performed. RT-PCR result showed that no apparent difference in PDS transcripts was perceived between WT and E17 either under standard growth conditions or induced conditions (Figure 4.5). In contrast, higher transcript levels of BKT and CHYb were found in E17 cells in response to 1-day high light treatment or induced by high concentration of glucose (30 g L-1) for 4 days. This result correlated well with the higher amounts of astaxanthin accumulated in E17 than in WT cells under high light stress (Figure 4.2A) or glucose induction conditions (Figure 4.2B). 100 Chpater 4. Molecular characterization of the Chlorella zofingiensis mutant E17 Figure 4.5 Transcript levels of PDS, BKT and CHYb in WT and E17 cells cultured under standard growth conditions (Ctr), high light induction for 1d (HL 1d) or 30 g L-1 glucose induction for 4d (30G 4d). Actin gene (ACT) was used as control. 4.5 Discussion C. zofingiensis synthesizes astaxanthin as secondary carotenoid that is stored in lipid vessicles outside the chloroplasts under stress conditions such as high light irradiation or nitrogen deficiency (Rise et al., 1994; Bar et al., 1995). A mutation in PDS revealed that this enzyme is one of the limiting steps of its biosynthetic pathway. An amino acid substitution (L516F) in PDS can not only make C. zofingiensis resistant to norflurazon (Figure 4.1), but also enhance the biosynthesis of TC and astaxanthin (Figure 4.2). Norflurazon-resistant phenotypes had been found before in Synechococcus PCC 7942 and Synechocystis sp. PCC 6803 mutants resulting from either a point mutation or a deletion in the upstream region of PDS gene (Chamovitz et al., 1991, 1993; Martinez-Ferez & Vioque, 1992). Point mutations in PDS give rise to a modified structure for the herbicide-binding site; whereas the deletion in regulating region causes up-regulation of PDS transcription and consequently a great increase of PDS protein (Chamovitz et al., 1993). Point mutations in PDS also resulted in plant mutants resistant to fluridone, another bleaching herbicide that also specifically 101 Chpater 4. Molecular characterization of the Chlorella zofingiensis mutant E17 inhibits the desaturation activity of PDS (Michel et al., 2004; Arias et al., 2005). Some mutations in PDS conferred cross-resistance to fluridone and norflurazon (Arias et al., 2006) which is not the case for E17 cells (data not shown). Norflurazon was reported to inhibit PDS by competition with the cofactor plastoquinone (Breitenbach et al., 2001). Therefore, bleaching herbicides including norflurazon can be regarded as plastoquinone analogs targetting the same binding region (Sandmann, 2002). E17 harbors a point mutation (C to T) in PDS, causing an amino acid substitution (L to F) at codon position 516. Surrounding the L residue is a conserved motif in PDS polypeptides from plants (Pecker et al., 1992; Scolnik & Bartley, 1993), algae (Harker & Hirschberg, 1997; McCarthy et al., 2004; Huang et al., 2008) and cyanobacteria (Chamovitaz et al., 1993). Previous mutations including the substitution of the L residue to arginine (R) in PDS had been found to confer norflurazon-resistant on Synechococcus sp. PCC 7942 but meanwhile lower the catalytic activity in phytoene desaturation and consequently reduce the production of downstream carotenoids (Sandmann et al., 1993). Similar results were obtained when the same mutation occurred in the PDS from H. pluvialis and C. zofingiensis (Steinbrenner & Sandmann, 2006; Huang et al., 2008). Thus the L residue may play a decisive role in the binding of herbicide and the binding of plastoquinone. In contrast to the substitution of L to R, the L to F exchange may favor the kinetics for plastoquinone binding resulting in higher PDS specific activity (Table 4.3). This leads to a higher TC accumulation in E17 (Figure 2). Transgenic H. pluvialis overexpressing an additional PDS gene also enhanced TC content (Steinbrenner & Sandmann, 2006). Determination of transcript levels of carotenogenic genes showed no changes for PDS in E17 (Figure 4.5). However, E17 accumulated higher levels not only of TC but also of astaxanthin than WT (Figure 4.2; Table 4.2). This is well-explained by the increased transcripts of BKT and CHYb in E17 (Figure 4.5). However, the regulatory mechanism leading to an up-regulation of both transcripts in E17 as a consequence of higher PDS activity remains open. 102 Chpater 4. Molecular characterization of the Chlorella zofingiensis mutant E17 In conclusion, the PDS gene of C. zofingienesis E17 harbors a point mutation which leads to one amino acid substitution and makes the mutated cells resistant to norflurazon. In contrast to all other mutations conferring norflurazon resistance, PDS in E17 shows increased specific activity leading to enhanced biosynthesis of carotenoids including the high-value astaxanthin. The mutated PDS gene may serve as a dominant selectable marker for genetic engineering of C. zofingiensis and even other green algae to enhance the biosynthesis of astaxanthin. 103 Chpater 5. Transformation of Chlorella zofingiensis with a phytoene desaturase gene for enhanced astaxanthin accumulation Chapter 5 Transformation of Chlorella zofingiensis with a phytoene desaturase gene for enhanced astaxanthin accumulation 5.1 Abstract PDS-L516F from the C. zofingiensis mutant E17 was revealed to show 31-fold greater resistance to norflurazon and have 29% higher desaturation activity compared with the unaltered PDS (Chapter 4). In this chapter, the transformation of C. zofingiensis with PDS-L516F gene was conducted, with an attempt to enhance the production of carotenoids. PDS-L516F gene was introduced into C. zofingiensis via biolistic approach. Transformants harboring the mutated PDS gene showed strong resistance to the herbicide norflurazon. The transformant P6 could accumulate 46.3% more astaxanthin than the WT. The enhanced accumulation of astaxanthin in the transformant was revealed to be related to the increase of PDS transcript. These results clearly show that the mutated PDS gene is a useful selectable marker that can be used for genetic engineering of carotenoid biosynthesis in C. zofingiensis. 5.2 Introduction Over the past decades, the carotenoid biosynthetic pathway and carotenogenic genes have been thoroughly investigated. It has greatly facilitated the genetic manipulation of carotenoid biosynthesis for special purposes, such as the enhancement of pre-existing carotenoids (Shewmaker et al., 1999; Romer et al., 2000; Steinbrenner & Sandmann, 2006) and the production of new pigments (Mann et al., 2000; Ralley et al., 2004; Morris et al., 2006; Jayaraj et al., 2008; 104 Chpater 5. Transformation of Chlorella zofingiensis with a phytoene desaturase gene for enhanced astaxanthin accumulation Zhong et al., 2008). While the engineering of carotenoid biosynthesis in higher plants has achieved great progress, it still remains at the very preliminary stage in many commercially important algae due to the shortage of suitable selectable genes for development of functional transformation systems. Modified endogenous genes as selectable markers proved effective in several algae (Dawson et al., 1997; Randolph-Anderson et al., 1998; Kovar et al., 2002; Steinbrenner & Sandmann, 2006). Phytoene desaturase is considered to catalyze a rate-limiting step in carotenoid biosynthesis (Chamovitz et al., 1993). It is also a target of various bleaching herbicides such as norflurazon and fluridone. Overexpression of PDSor crtI-type phytoene desaturase gene could result in the elevated production of carotenoids in transgenic plants and algae (Misawa et al., 1993; Romer et al., 2000; Steinbrenner & Sandmann, 2006). In chapter 4, the PDS gene of C. zofingiensis mutant E17 was isolated and characterized. This PDS gene, with a codon change from L to F at position 516 (Figure 5.1A), was revealed to be resistant to norflurazon and have an improved desaturation activity. Since C. zofingiensis is very sensitive to this herbicide, PDS-L516F gene may be adopted as a dominant selectable marker for the transformation of C. zofingiensis. It is expected that transformants harboring the PDS-L516F gene would synthesize more carotenoids than WT. This chapter described the nuclear transformation of C. zofingiensis with PDS-L516F gene as a selectable marker. Putative transformants showed noflurazon resistance, increased PDS expression and elevated accumulation of carotenoids including astaxanthin. 105 Chpater 5. Transformation of Chlorella zofingiensis with a phytoene desaturase gene for enhanced astaxanthin accumulation Figure 5-1 (A) PDS from the norflurazon-resistant C. zofingiensis mutant E17 showing the codon change from Leu to Phe at position 516 and the chloroplast transit peptide (TP) identified by the ChloroP program. (B) Map of C. zofingiensis transformation vector pBlue-PDS-L516F with restriction sites indicated. The E17 PDS cDNA with its promoter and terminater (PDS-L516F) is inserted into the vector pBluescript SKII (+). The construct also contains the ampicillin resistance gene, the E. coli origin of replication (ColE1). 5.3 Materials and methods 5.3.1 Algal strain and culture conditions The maintenance and inoculation of C. zofingiensis were described in 106 Chpater 5. Transformation of Chlorella zofingiensis with a phytoene desaturase gene for enhanced astaxanthin accumulation 2.3.1. For herbicide treatment, the cell cultures of WT and transformants were grown in Kuhl medium for 4 days and then inoculated into the same medium containing 0.5 μM norflurazon for growth under continuous illumination of 25 µmol photon m-2 s-1. For induction of astaxanthin accumulation, the 4-day cultures mentioned above were inoculated into fresh Kuhl medium containing 30 g L-1 glucose and maintained in the dark. 5.3.2 Construction of the transformation vector pBlue-PDS-L516F and transformation protocol A 2.4-kb promoter and a 0.6-kb terminator of PDS were amplified, digested (HindIII/EcoRI and BamHI/XbaI, respectively) and inserted sequentially into the corresponding restriction sites of the pBluescript SKII(+) vector (Stratagene), followed by the insertion of PDS-L516F excised from pUC-czPDS-L516F (Chapter 4), giving rise to the transformation vector pBlue-PDS-L516F (Figure 5.1B). C. zofingienesis cells grown in Kuhl medium for 3 days under standard growth conditions were collected and re-suspended to a density of around 1 × 108 cells mL-1 in liquid medium. One milliliter of the concentrated cells was used for each bombardment and plated on filters on Kuhl plates. For transformation, the Biolistic PDS-1000/He system was employed (Bio-Rad, Hercules, CA, USA). Fifty microliters of a Gold particle solution (0.6 μm, 60 mg mL-1) was mixed with 5 μL of plasmid solution (1 μg μL-1), 50 μL of 2.5 M CaCl2, and 20 μL of 0.1 M spermidine (Sigma). The mixture was incubated at room temperature for 10 min and centrifuged for 10 s. The pellet was washed once with 70% ethanol and twice with 100% ethanol, and re-suspended in 50 μL ethanol. Each 10 μL of the DNA-coated gold particles was layered on a macrocarrier. Plates were bombarded from a distance of 6 cm under vacuum of 28 mm Hg using 1, 350-lb in-2 rupture disks. After a 24-h recovery, the bombarded cells were spread on Kuhl plates 107 Chpater 5. Transformation of Chlorella zofingiensis with a phytoene desaturase gene for enhanced astaxanthin accumulation containing 0.5 μM norflurazon. Colonies appearing after 3 to 4 weeks were picked up and re-streaked three times on selective Kuhl plates. 5.3.3 Genomic DNA and RNA isolation Genomic DNA and RNA isolation were described in 2.3.2 5.3.4 PCR determination of transformants To distinguish the introduced PDS-L516F from genomic PDS gene, a pair of PCR primers that locates at two individual exons interrupted by a 0.27-kb intron were designed, which amplified 0.62- and 0.35-kb products for genomic PDS and PDS-L516F genes respectively (Table 5.1). Primers for amplification of a 0.22-kb fragment of ampicillin resistance gene were also employed (Table 5.1). Cell samples harvested from 4-day cultures under illumination of 25 µmol photon m-2 s-1 were used for determination of PDS expression. Table 5.1 Primers for amplification of PDS and ampicillin resistant genes Gene Primers (5′-3′) Product size (kb) PDS Forward: GATTGGGCGGAGTGATGAGG 0.62 (0.35) (PDS-L516F) Reverse: CTGTCGCTAATGCGGGTTTC Ampicillin Forward: AAGCCATACCAAACGACGAG resistant gene Reverse: GTCGTGTAGATAACTACGATA 5.3.5 Cell dry weight determination Cell dry weight determination was described in 3.3.5. 108 0.22 Chpater 5. Transformation of Chlorella zofingiensis with a phytoene desaturase gene for enhanced astaxanthin accumulation 5.3.6 Extraction and analysis of pigments The extraction and pigment analysis were described in 3.3.7. 5.3.7 RT-PCR assay RT-PCR assay was described in 3.3.8 except that the amplification cycles for PDS were lowered to 22. 5.4 Results 5.4.1 Analysis of C. zofingiensis transformants After transformation of C. zofingiensis with the vectors pBlue-PDS-L516F and pBlue-PDS by particle bombardment and a recovering period, the cells were spread on selective Kuhl plates containing 0.5 μM norflurazon. After 3 to 4 weeks of growth, colonies appeared in samples transformed with pBlue-PDS-L516F, but not in samples transformed with pBlue-PDS that harbors the WT PDS gene. The colonies were re-streaked three times on selective plates and then inoculated into liquid media. To determine the stable nuclear integration of PDS-L516F gene, PCR analysis of the transformants was performed with primers specific to the PDS gene and the ampicillin resistance cassette of the transformation vector. The endogenous PDS gene was detected in the WT and all transformnts (Figure 5.2A, upper band with a size of 0.62 kb). The transformants examined had an additional shorter band amplified from the PDS-L516F gene (Figure 5.2A, lower band with a size of 0.35 kb). PCR determination of the ampicillin resistance cassette was also conducted and shown in Figure 5.2B. All transformants except P3 showed a 0.22-kb band. This finding suggested that some parts of the transformation vector may lose during its 109 Chpater 5. Transformation of Chlorella zofingiensis with a phytoene desaturase gene for enhanced astaxanthin accumulation integration into the nuclear genome of C. zofingiensis. To survey the expression of transgene in transformants, RT-PCR analysis was performed. Cell samples were harvested from 4-day cultures under illumination of 25 µmol photon m-2 s-1. Compared with WT cells that exhibited a basal expression of the PDS gene, transformed cells contained increased levels of PDS transcripts (Figure 5.2C). Figure 5-2 PCR determination of C. zofingiensis transformants. Primers for amplification of PDS and ampicillin resistance genes were shown in Table 5.1. (A) PCR amplification of PDS-L516F gene in transformants. (B) PCR amplification of ampicillin resistant gene in transformants. (C) RT-PCR determination of PDS gene expression in transformants. For comparison, total RNA was equally loaded (lower panel). To obtain further information about the expression of transgenic PDS-L516F, and the functionality of the 2.4-kb promoter and 0.6-kb terminator of PDS gene, cDNA synthesis and subsequent sequencing of the PDS transcript pool were conducted. No additional mutations were observed in the PDS cDNA except for a mixture of CTT and TTT at codon position 516 for transformants. These findings indicate that the 2.4-kb promoter region of PDS gene is sufficient to drive the expression of transgene in C. zofingiensis. 110 Chpater 5. Transformation of Chlorella zofingiensis with a phytoene desaturase gene for enhanced astaxanthin accumulation 5.4.2 Resistance of transformants to norflurazon To survey the effect of norflurazon on cell growth and carotenoid accumulation, time courses of biomass, TC and astaxanthin contents of the WT and transformants were conducted. Cell samples treated with 0.5 μM norflurazon or without norflurazon were cultured under the continuous light illumination of 25 µmol photon m-2 s-1. In the presence of 0.5 μM norflurazon, the growth of WT was severely inhibited, as indicated by the much lower cell biomass (Figure 5.3A, left); the biosyntehsis of TC and astaxanthin was also severely inhibited and got completely blocked as the exposure time to norflurazon prolonged (Figure 5.3B and C, left). In contrast, the growth of the transformant P6 was not sensitive to norflurazon, as indicated by the unaffected cell biomass (Figure 5.3A, right); the accumulation of TC and astaxanthin was only slightly alleviated (Figure 5.3B and C, right). At the expense of colored carotenoids, phytoene was found to accumulate in both norflurazon-treated WT and P6 cells, together with the concurrent decrease of chlorophylls, yet P6 was less affected (Table 5.2). These results indicate that the transformant P6 exhibited strong resistance to norflurazon. Similar to P6, other transformants were also resistant to norflurazon (data not shown). In addition, the transformation stability was analyzed. After more than 50 times of subculture under non-selective conditions, the transformants showed no loss of norflurazon resistance and stable integration of transgene in the genome (data not shown). 111 Chpater 5. Transformation of Chlorella zofingiensis with a phytoene desaturase gene for enhanced astaxanthin accumulation Figure 5.3 The effect of norflurazon on biomass (A), TC (B) and astaxanthin (C) contents of WT (left) and transformant P6 (right). Cells were cultured under illumination of 25 µmol photon m-2 s-1. Table 5.2 Content of colored carotenoids, phytoene and chlorophyll in algal cells cultured for 4 days with (+) or without (-) 0.5 μM norflurazaon Pigments WT P6 transformant (mg g-1 dry weight) - + - + Colored carotenoids 1.79 ± 0.07 0.12 ± 0.01 1.92 ± 0.08 1.51 ± 0.05 Nd a 0.89 ± 0.03 Nd 0.19 ± 0.01 5.84 ± 0.23 0.94 ± 0.04 5.98 ± 0.31 3.39 ± 0.14 Phytoene Chlorophylls a Nd, not detected 112 Chpater 5. Transformation of Chlorella zofingiensis with a phytoene desaturase gene for enhanced astaxanthin accumulation 5.4.3 Enhanced biosynthesis of astaxanthin in P6 induced by glucose Since glucose can boost C. zofingiensis to synthesize carotenoids including astaxanthin (Ip & Chen, 2005; Sun et al., 2008), it is interesting to compare the time courses of TC and astaxanthin accumulation in heterotrophic WT and P6 cells cultured with glucose, which is shown in Figure 5.4A. Upon induction by glucose, the TC and astaxanthin contents in WT cells remained almost unchanged during the first 2 days. Thereafter, a significant increase in both TC and astaxanthin contents was observed. The transformant P6 showed the same induction pattern of TC and astaxanthin as that of WT. However, P6 produced apparently greater amounts of carotenoids than the WT during the whole induction period. The carotenoid contents of WT and P6 induced with glucose for 5 days are shown in Table 5.3. Lutein, astaxanthin and adonixanthin were the major carotenoids. Significant higher amounts of β-carotene, lutein, zeaxanthin, canthaxanthin, adonixanthin and astaxanthin were observed to accumulate in P6 cells. The TC and astaxanthin contents of P6 were 2.45 mg g-1 and 0.64 mg g-1, 20.7% and 46.3% higher than that of WT respectively. Correlated with the higher content of astaxanthin, P6 cells exhibited a deeper yellow-orange color than WT (Figure 5.4B). 113 Chpater 5. Transformation of Chlorella zofingiensis with a phytoene desaturase gene for enhanced astaxanthin accumulation Figure 5.4 Comparison of WT and P6 cultured with 30 g L-1 glucose in the dark. (A) Time course of accumulation of TC (square) and astaxanthin (circle) in WT (open) and P6 (filled). (B) Algal cells of WT and P6 from 5-day cultures. Table 5.3 Pigment profiles in WT and P6 cells cultured with 30 g L-1 glucose in the dark for 5 days a TC Chl (mg g-1) (mg g-1) WT 2.03 ± 0.11 P6 2.45 ± 0.13 a Carotenoid composition (μg g-1) Neo Vio Ant Ast Ado Lut Zea Can β-Car 3.23 ± 0.15 69 ± 2.5 32 ± 1.4 73 ± 2.8 434 ± 24 366 ± 16 542 ± 24 224 ± 14 205 ± 13 86 ± 4.9 2.94 ± 0.13 61 ± 3.3 35 ± 1.8 61 ± 3.3 635 ± 29 438 ± 25 623 ± 38 256 ± 17 231 ± 11 109 ± 5.7 Data were expressed as mean values of three independent experiments. TC, total carotenoids; Chl, Chlorophylls; Neo, neoxanthin; Vio, violaxanthin; Ant, antheraxanthin; Ast, astaxanthin; Ado, adonixanthin; Lut, lutein; Zea, zeaxanthin; Can, canthaxanthin; β-Car, β-carotene 114 Chpater 5. Transformation of Chlorella zofingiensis with a phytoene desaturase gene for enhanced astaxanthin accumulation 5.4.4 Transcription analysis of carotenogenic genes It has been reported that the elevated biosynthesis of carotenoids including astaxanthin in C. zofingiensis by glucose induction was correlated to the increased transcript levels of carotenogenic genes (Li et al., 2009). To further examine if the elevated production of TC and astaxanthin by P6 as compared to the WT was attributed to the up-regulation of PDS and/or other carotenogenic genes, the transcript levels of PDS, BKT and CHYb were semi-quantified by using the RT-PCR approach. Cells samples induced for 0 day, 2 days and 4 days were employed. At all stages examined, P6 contained a much higher amount of PDS transcripts than WT (Figure 5.5). Although no apparent differences in transcripts of BKT and CHYb between WT and P6 were observed for 0-day cell cultures, significant higher transcript levels of these two genes were found in P6 after induction of 2 days and 4 days (Figure 5.5). This result correlated well to the higher amounts of TC including astaxanthin accumulated in P6 cells in response to glucose induction (Figure 5.4A). Figure 5.5 Transcript levels of PDS, BKT and CHYb in WT and P6 cells cultured in the dark with 30 g L-1 glucose for 0 day (0 D), 2 days (2 D) and 4 days (4 D). Actin gene (ACT) was used as control. 115 Chpater 5. Transformation of Chlorella zofingiensis with a phytoene desaturase gene for enhanced astaxanthin accumulation 5.5 Discussion C. zofingiensis represents a typical fast growing alga that can be heterotrophically cultivated for astaxanthin production (Ip & Chen, 2005). The enhanced cellular astaxanthin content, which may be achieved through genetic engineering of the carotenoid biosynthesis, will make C. zofingiensis more attractive for commercial use. At present, no appropriate transformation system is available for the alga. The availability of suitable promoters, terminators and promising reporter or resistance genes proved crucial for the development of a transformation system of algae (Cerutti et al., 1997; Schroda et al., 2000; Poulsen & Kroger, 2005; Steinbrenner & Sandmann, 2006). It was reported in chapter 4 that the PDS-L516F gene isolated from the C. zofingiensis mutant E17 encoded a desaturase having 31-fold greater resistance to norflurazon than the WT one. The PDS-L516F gene can therefore serve as a selectable marker for the transformation of C. zofingiensis. The integration of the transformation vector pBlue-PDS-L516F was investigated by PCR analysis (Figure 5.2A and B). Using the primers specific to two individual exons of PDS gene revealed that all transformants except the WT had an additional band indicating the presence of PDS-L516F in the nuclear genome. The integrated transgene conferred enhanced PDS expression on transformants, as indicated by the increased amounts PDS transcripts (Figure 5.2C). This was further confirmed by the analysis of the total PDS gene transcripts, which revealed the presence of a mixture of WT and mutated PDS transcripts. These results also suggested that the expression cassette of PDS gene drove efficiently the expression of PDS-L516F cDNA and that the endogenous PDS gene was intact. The expression of PDS-L516F gene not only conferred norflurazon resistance on transformants (Figure 5.3), but also influenced their carotenoid metabolism. Carotenoid analysis showed that the transformant P6 could accumulate higher amount of TC than WT when cultured with glucose in the dark 116 Chpater 5. Transformation of Chlorella zofingiensis with a phytoene desaturase gene for enhanced astaxanthin accumulation (Figure 5.4A). This is in accordance with the previous findings that the introduction of a mutated PDS gene resulted in elevated production of carotenoids in transgenic H. pluvialis (Steinbrenner & Sandmann, 2006). In addition to the primary carotenoids, secondary carotenoids such as canthaxanthin, adonixanthin and astaxanthin were also found in higher amounts in P6 (Table 5.3). After 5 days of glucose induction, P6 produced 46.3% more astaxanthin than WT did. It is possible that the increased expression of PDS gene results in an increase in the flux of colored carotenoids, which in turn up-regulate the transcription of such carotenogenic genes as BKT and CHYb, leading to the enhanced synthesis of astaxanthin (Figure 5.4 and 5.5). Similar phenomenon was observed for transgenic tomato plants in which overexpression of a crtI-type phytoene desaturase caused up-regulation of ζ-carotene desaturase and lycopene β-cyclase genes and elevation of β-carotene synthesis (Romer et al., 2000). These results suggest that phytoene desaturation is a rate-limiting step for carotenoid biosynthesis in C. zofingiensis. The transformant P6 harbored at least one copy of the PDS-L516F gene from the C. zofingiensis mutant E17. Compared with E17, P6 contained much higher amount of PDS transcripts, as indicated by the RT-PCR analysis (Figure 5.6A). However, no significant difference in both TC and astaxanthin contents was observed between E17 and P6 (Figure 5.6B). This might be due to the involvement of complex mechanisms in carotenoid biosynthesis, which restricts carotenoid flux within a certain range when genetic engineering of the phytoene desaturation step only is implemented. Considering the presence of substantial amounts of the end product canthaxanthin and the intermediate product adonixanthin (Table 5.3), CHYb may not accept canthaxanthin as substrate to produce astaxanthin and BKT might not efficiently convert adonixanthin to astaxanthin in C. zofingiensis. Therefore, the manipulation of specific astaxanthin biosynthetic steps by introducing a carotenoid hydroxylase that can convert canthaxanthin to astaxanthin and a carotenoid ketolase that can catalyze the efficient conversion of adonixanthin to astaxanthin, should represent a feasible 117 Chpater 5. Transformation of Chlorella zofingiensis with a phytoene desaturase gene for enhanced astaxanthin accumulation strategy for enhanced accumulation of astaxanthin (Figure 1.16). In conclusion, the results reported here clearly demonstrate that the PDS-L516F gene can be adopted as a dominant selectable marker for stable nuclear transformation of C. zofingiensis. The transformants overexpressing the PDS gene could enhance the flux of colored carotenoids, providing a proof of concept for genetic engineering of the carotenoid biosynthesis in C. zofingiensis toward elevated astaxanthin production. Figure 5.6 Comparison of E17 and P6 cultured with 30 g L-1 glucose in the dark. (A) Transcript levels of PDS in E17 and P6 cells cultured for 2 days (2 D) and 4 days (4 D). Actin gene (ACT) was used as control. (B) Carotenoid accumulation in E17 and P6 cells cultured for 4 days (4 D) and 6 days (6 D). 118 PART III POTENTIAL ASSESSMENT OF CHLORELLA ZOFINGIENSIS AS A BIODIESEL FEEDSTOCK 119 Chapter 6. Differential lipid and fatty acid profiles of photoautotrophic and heterotrophic Chlorella zofingiensis: assessment of algal oils for biodiesel prodoction Chapter 6 Differential lipid and fatty acid profiles of photoautotrophic and heterotrophic Chlorella zofingiensis: assessment of algal oils for biodiesel production 6.1 Abstract Profiles of algal lipids and fatty acids, which depend on species and culture conditions, are important data to evaluate the oils for biodiesel production. The objective of this study was to document and compare the lipid class and fatty acid composition of the green microalga Chlorella zofingiensis cultivated under photoautotrophic and heterotrophic conditions. Compared with photoautotrophic cells, a 900% increase in lipid yield was achieved in heterotrophic cells fed with 30 g L-1 glucose. Furthermore heterotrophic cells accumulated predominantly neutral lipids (NL) that accounted for 79.5% of total lipids, with 88.7% of NL being triacylglycerol (TAG); whereas photoautotrophic cells contained mainly the membrane lipids glycolipids (GL) and phospholipids (PL). Together with the much higher content of oleic acid (C18:1, 35.2% of total fatty acids), oils from heterotrophic C. zofingiensis appear to be more feasible for biodiesel production. The study highlights the possibility of using heterotrophic algae for producing high quality biodiesel. 120 Chapter 6. Differential lipid and fatty acid profiles of photoautotrophic and heterotrophic Chlorella zofingiensis: assessment of algal oils for biodiesel prodoction 6.2 Introduction Energy is an indispensable factor to sustain our economic growth and living standard. Up to date, fossil-derived fuels such as coal, petroleum and natural gas have still served as the main global energy sources. The growing consumption of fossil fuels, however, has caused many environmental problems that threaten our ecosystem (Jain & Sharma, 2010; Lim & Teong, 2010). Furthermore, fossil fuels are recognized as unsustainable due to their depleting supplies (Chisti, 2008; Shafiee & Topal, 2009). Thus, clean and renewable energy has to be explored. Biofuels, especially biodiesel, are carbon neutral, contributing less emission of gaseous pollutants, and are therefore environmentally beneficial (Ma & Hanna, 1999; Fukuda et al., 2001; Knothe, 2005b; Gerpen, 2005; Meher et al., 2006; Hu et al., 2008). Biodiesel is chemically composed of fatty acid methyl ester (FAME) that is traditionally derived from transesterification of vegetable oils or animal fats (Lang et al., 2001; Al-Widyan & Al-Shyoukh, 2002; Zhang et al., 2003; Demirbas, 2005). Microalgae have been cited as promising feedstocks for biodiesel production because of their rapid growth rate and high intracellular content of lipids (Chisti, 2007; Hu et al., 2008). Two systems are commonly used to cultivate algae: the open system (open raceway ponds) and the closed system (photobioreactors). As high oil strains generally grow slower than low oil strains, open systems can only be used for culturing “extremophile” that can tolerate extreme conditions (e.g., high salinity or alkalinity) in which other strains cannot survive (Vasudevan & Briggs, 2008). Unfortunately no such strains with high content of oils have been isolated. Enclosed photobioreactors are desirable for maintaining pure culture and fast growth of oil-rich algae. However, as algae only accumulate large amounts of oil under stresses (mostly nutrient restrictions) that also limit cell growth, it is difficult to balance the growth and oil production of an alga with current photobioreactors. These challenges need to be addressed before profitable biodiesel can be produced from algae. 121 Chapter 6. Differential lipid and fatty acid profiles of photoautotrophic and heterotrophic Chlorella zofingiensis: assessment of algal oils for biodiesel prodoction C. zofingiensis is a particular green alga in that it can grow well photoautotrophically as well as heterotrophically (Orosa et al., 2000; Ip & Chen, 2005; Sun et al., 2008). High cell biomass (up to 53 g L-1) of the alga could be achieved by using a glucose fed-batch fermentation strategy (Sun et al., 2008). Furthermore, C. zofingiensis also accumulated high amounts of the secondary ketocarotenoid astaxanthin when grown with glucose as the sole carbon and energy source (Ip & Chen, 2005; Sun et al., 2008). Phylogenetic relatives of C. zofingiensis, such as Chlorella vulgaris and Chlorella protothecoides were reported to accumulate high levels of lipids when cultivated under heterotrophic conditions (Miao & Wu, 2004; Liu et al., 2008; Hsieh & Wu, 2009). The fact that carotenoid biosynthesis increases with oil synthesis further raises the possibility of using C. zofingiensis as a cell factory for algal oil. However, knowledge on Chlorella species to accumulate lipids and fatty acids under different growth modes remains largely unknown. In addition, the feasibility of various algal oils for producing high quality biodiesel need to be assessed. The objective of this study was to document and compare the lipid class and fatty acid composition of C. zofingiensis cultivated under photoautotrophic and heterotrophic conditions so as to assess its feasibility for biodiesel production. 6.3 Methods and materials 6.3.1 Algal strain and culture conditions The maintenance and inoculation of C. zofingiensis were described in 2.3.1. For photoautotrophic growth, the seed cells were inoculated into the fresh medium and allowed to grow under continuous illumination of 30 µmol photon m-2 s-1 with orbital shaking at 150 rpm. For heterotrophic growth, the seed cells were inoculated into the medium containing 30 g L-1 glucose and cultured in the dark with orbital shaking at 150 rpm. 122 Chapter 6. Differential lipid and fatty acid profiles of photoautotrophic and heterotrophic Chlorella zofingiensis: assessment of algal oils for biodiesel prodoction 6.3.2 Determination of glucose concentration, nitrate concentration, cell dry weight and specific growth rate The cells were centrifuged at 3,800 g for 5 min. Glucose concentration and nitrate concentration in the supernatant were determined according to Miller (1959) and Elton-Bott (1979), respectively. Cell dry weight determination was described in 3.3.5. The specific growth rate (µ) at the exponential phase was calculated according to the equation µ = (ln X2 − ln X1) / (t2 − t1), where X2 and X1 are the cell dry weight concentration (g L−1) at time t2 and t1, respectively. 6.3.3 Lipid extraction and analysis Cells were harvested and lyophilized for lipid extraction and analysis. Total lipids were extracted from 200 mg of lyophilized cells with a solvent mixture of chloroform, methanol and water (2:1:0.75, by vol.) according to the modified Folch procedure (Christie, 2003). The extract was dried in a rotary evaporator, and then weighed, re-suspended in chloroform, and finally stored at -20 °C under nitrogen gas to prevent lipid oxidation. Lipids were separated into neutral lipids (NL), glycolipids (GL) and phospholipids (PL) using solid-phase extraction (Christie, 2003). A 500 mg Sep-PakTM cartridge of silica gel (Waters) was first conditioned by elution with 5 ml of chloroform, and about 50 mg of lipids were then applied to the column. Elution with 10 ml of solvent in the order of chloroform, acetone and methanol yielded NL, GL and PL, respectively. Each fraction was dried under a stream of nitrogen gas, weighted and then re-suspended in 0.1 mL of chloroform. The NL fraction was subjected to one-dimensional thin-layer chromatography (TLC) for lipid class separation and identification, using TLC plates (20 × 20 cm) coated with silica gel 60 (Merck, Whitehouse Station, NJ, USA) (Fan et al., 2007). Plates were activated in an oven at 100 °C for 2 h prior to 123 Chapter 6. Differential lipid and fatty acid profiles of photoautotrophic and heterotrophic Chlorella zofingiensis: assessment of algal oils for biodiesel prodoction use. The solvent mixture hexane/diethyl ether/acetic acid (70:30:1, by vol.) was used. After co-chromatography with pure standards (Sigma), bands of lipid classes were stained with 2,7-dichlorofluorescein (Sigma) and visualized under UV light. 6.3.4 Fatty acid analysis Fatty acid methyl esters (FAMEs) were prepared by direct transmethylation with sulphuric acid in methanol (Christie, 2003). The FAMEs were analyzed by using an HP 6890 capillary gas chromatography (Hewlett-Packard, Palo Alto, CA) equipped with a flame ionization detector (FID) and a HP-INNOwax capillary column (30 m × 0.32 mm) (Agilent Technologies, Inc., Wilmington, DE). Nitrogen was used as carrier gas. Initial column temperature was set at 170 °C, which was progressively raised to 230 °C at 1 °C/min. The injector was kept as 250 °C with an injection volume of 2 µL under splitless mode. The FID temperature was set at 270 °C. FAMEs were identified by chromatographic comparison with authentic standards (Sigma). The quantities of individual FAME were estimated from the peak areas on the chromatogram using heptadecanoic acid (Sigma) as the internal standard. 6.4.5 Calculation of iodine value The iodine value of algal oils was calculated according to AOCS recommended practice Cd 1c-85, which estimates the grams of halogen absorbed by 100 g of oil. 124 Chapter 6. Differential lipid and fatty acid profiles of photoautotrophic and heterotrophic Chlorella zofingiensis: assessment of algal oils for biodiesel prodoction 6.5 Results 6.5.1 Growth characteristics and fatty acid accumulation C. zofingiensis can grow photoautotrophically and heterotrophically utilizing sugars as the carbon and energy sources (Del Campo et al., 2004; Ip & Chen, 2005; Sun et al., 2008). The growth parameters of the algal cells grown photoautotrophically or heterotrophically in batch cultures were characterized here. As shown in Table 6.1, photoautotrophic cells grew slowly indicated by the low specific growth rate (0.235 d-1) and cell biomass (1.9 g L-1). In contrast, the alga grew fast under heterotrophic conditions supplemented with 30 g L-1 glucose, with a specific growth rate of 0.769 d-1 and a cell biomass concentration of 9.7 g L-1, which are 227% and 411% higher than those of the photoautotrophic cells respectively. The glucose utilization and growth yield coefficient based on glucose by heterotrophic cells were revealed to be 77.8% and 0.402 g/g, respectively. In addition, heterotrophic cells consumed nitrated from the medium rapidly (Figure 6.1A). Table 6.1 Growth kinetic parameters of C. zofingiensis in photoautotrophic and heterotrophic batch cultures a Parameters b Photoautotrophic Heterotrophic 0.235 ± 0.014 0.769 ± 0.046 Xmax (g L-1) 1.9 ± 0.11 9.7 ± 0.33 Yx/gu (g g-1) — 0.402 ± 0.012 Glucose utilization (%) — 77.8 ± 4.2 μ (d-1) a C. zofingiensis Data are expressed as mean ± standard deviation of triplicates. b μ, specific growth rate; Xmax, maximum biomass concentration; Yx/glu, growth yield coefficient based on glucose. 125 Chapter 6. Differential lipid and fatty acid profiles of photoautotrophic and heterotrophic Chlorella zofingiensis: assessment of algal oils for biodiesel prodoction Figure 6.1 Cell growth (down triangle) and nitrate consumption (up triangle) by heterotrophic C. zofingiensis (A) and accumulation of TFA (square) and oleic acid (circle) in autotrophic (open) and heterotrophic (solid) algal cells (B). To investigate the effect of growth modes on fatty acid accumulation, the time courses of total fatty acids (TFA) and oleic acid (C18:1) accumulated in C. zofingiensis grown photoautotrophically or heterotrophically were surveyed here. Figure 6.1B shows that photoautotrophic cells maintained basal amounts of TFA and oleic acid (15.8% and 3.23% of dry weight on day 14, respectively) during the time surveyed. In contrast, a steady increase in both TFA and oleic acid was 126 Chapter 6. Differential lipid and fatty acid profiles of photoautotrophic and heterotrophic Chlorella zofingiensis: assessment of algal oils for biodiesel prodoction observed in heterotrophic cells during the culture time, which reached up to 42.1% and 15.2% of dry weight respectively on the 14th day. Similar to plants, microalgae synthesize fatty acids in the chloroplast using a single set of enzymes, of which acetyl-CoA carboxylase (ACCase) is the key one in regulating fatty acid synthesis rates while stearoyl ACP desaturase adds the first double bond to acyl chain and plays an important role in determining the ratio of unsaturated fatty acids to saturated ones (Ohlrogge & Jaworski, 1997). Preliminary results showed that glucose could triggered the strong up-regulation of ACCase and stearoyl ACP desaturase genes in C. zofingiensis (data not shown), which may enhance the flux of both TFA and unsaturated fatty acids and thus the accumulation of TFA and oleic acid. 6.5.2 Lipid class composition To further survey the lipid profiles of the alga, extraction and characterization of the lipids from cells grown photoautotrophically or heterotrophically for 14 days were conducted. The results were shown in Figure 6.2. Heterotrophic cells could accumulate lipids up to 51.1% of dry weight, which is nearly 100% higher than that from photoautotrophic cells (25.8% of dry weight) (Figure 6.2A). Total lipids were mainly separated into NL, GL and PL using solid-phase extraction (Figure 6.2B). In photoautotrophic cells, membrane lipids, namely GL sand PL, were the major lipid classes which altogether accounted for 70.6% of total lipids, while the storage lipids NL accounted for only 29.4% of the total lipids (Figure 6.2B). In contrast, heterotrophic cells produced predominantly NL which represented 80.9% of total lipids. Thus, photoautotrophic and heterotrophic cells exhibited a great discrepancy of NL proportions (Figure 6.2B). Unlike photoautotrophic cells, heterotrophic cells can channel excessive carbon for the biosynthesis of storage lipids, for example NL, instead of converting carbon into membrane lipids for building photosynthetic apparatus. 127 Chapter 6. Differential lipid and fatty acid profiles of photoautotrophic and heterotrophic Chlorella zofingiensis: assessment of algal oils for biodiesel prodoction Figure 6.2 Lipid content and fractionation of C. zofingiensis. (A) Lipid content of photoautotrophic and heterotrophic cells. (B) Distribution of NL, GL, and PL in lipids of photoautotrophic (white column) and heterotrophic (gray column) cells. (C) Distribution of NL subclasses of photoautotrophic (white column) and heterotrophic (gray column) cells. NL, neutral lipids; GL, glycolipids; PL, phospholipids; SE, steroid ester; TAG, triacylglycerol; FFA, free fatty acids; DAG, diacylglycerol; MAG, monoacylglycerol. The NL fraction was separated by TLC into individual subclasses for further analysis. For heterotrophic cells, TAG was the most abundant component which accounted for 88.7% and 70.5% of NL and total lipids respectively (Figure 6.2C). Diacylglycerol (DAG, 25.2 mg g-1 dry weight), monoacylglycerol (MAG, 128 Chapter 6. Differential lipid and fatty acid profiles of photoautotrophic and heterotrophic Chlorella zofingiensis: assessment of algal oils for biodiesel prodoction 9.1 mg g-1 dry weight), sterol and free fatty acids were found in small proportions in heterotrophic cells (Figure 6.2C). As the key metabolites in TAG biosynthesis, DAG and MAG were also found in low amount in other heterotrophically grown algae (Alonso et al., 2000; Chen et al., 2007; Fan et al., 2008). Compared with heterotrophic cells, photoautotrophic cells had a lower TAG content (65.9% of NL) but higher contents of DAG and MAG (Figure 6.2C). Although TAG was the major component of NL in photoautotrophic cells, its cellular content is merely 49.3 mg g-1 dry weight, which was much lower than that obtained from heterotrophic cells (360.3 mg g-1 dry weight). 6.5.3 Fatty acid composition of individual lipid classes Fatty acid composition is an important parameter to assess the feasibility of algal oils for biodiesel production. Therefore here the fatty acid profiles of individual lipid classes from both photoautotrophic and heterotrophic cells were investigated and are presented in Table 6.2. C16:0, oleic acid and C18:2 were found to be the major fatty acids, which together accounted for 65.3% or 77.6% of TFA in photoautotrophic or heterotrophic cells respectively (Table 6.2). However, saturated fatty acids (SFA), monounsaturated fatty acids (MUFA), and polyunsaturated fatty acids (PUFA) displayed a significant interactive effect of growth modes. For example, photoautotrophic cells contained a higher percentage of PUFA (49.6%) but a lower MUFA (20.1%) as compared with that from heterotrophic cells (39.1% and 37.4% respectively). The higher MUFA content in heterotrophic cells is attributed predominantly to the higher proportion of oleic acid (Table 6.2). Based on the fatty acid profile, the calculated iodine value of oils from photoautotrophic and heterotrophic cells were 133.5 g I2/100 g and 117.9 g I2/100 g, respectively. 129 Chapter 6. Differential lipid and fatty acid profiles of photoautotrophic and heterotrophic Chlorella zofingiensis: assessment of algal oils for biodiesel prodoction Table 6.2 Fatty acid composition of individual lipid class of C. zofingiensis (% of TFA) a Photoautotrophic cells Heterotrophic cells Fatty acids TL TAG DAG MAG SE FFA GL PL TL TAG DAG MAG SE FFA GL PL 16:0 26.6 ± 1.1 25.1 ± 1.4 20.3 ± 0.8 25.9 ± 1.4 29.0 ±1.2 25.6 ±1.1 20.6 ± 0.9 37.4 ±2.3 22.2 ± 1.0 21.8 ± 1.2 20.2 ± 0.8 21.7 ± 0.7 50.5 ± 2.2 18.9 ± 0.7 27.4 ± 1.5 43.1 ± 1.7 16:1 2.2 ± 0.1 2.0 ± 0.1 9.8 ± 1.1 1.7 ± 0.1 3.8 ± 0.2 2.8 ± 0.2 1.0 ± 0.0 1.0 ± 0.1 1.7 ± 0.1 1.6 ± 0.1 4.5 ± 0.3 2.3 ± 0.1 6.6 ± 0.3 2.2 ± 0.1 2.3 ± 0.1 1.6 ± 0.1 16:2 7.7 ± 0.2 7.8 ± 0.3 11.1 ± 0.4 5.1 ± 0.3 4.6 ± 0.3 5.2 ± 0.2 8.0 ± 0.5 7.2 ± 0.4 8.3 ± 0.4 8.2 ± 0.3 9.5 ± 0.4 5.8 ± 0.2 4.7 ± 0.3 13.0 ± 0.5 19.1 ± 0.7 7.0 ± 0.2 16:3 6.6 ± 0.3 5.5 ± 0.3 10.3 ± 0.6 4.9 ± 0.3 31.5 ± 2.1 10.0 ± 0.7 7.2 ± 0.3 5.2 ± 0.3 2.1 ± 0.1 2.3 ± 0.1 3.2 ± 0.1 2.9 ± 0.1 6.2 ± 0.2 13.1 ± 0.5 3.4 ± 0.2 1.0 ± 0.1 16:4 1.1 ± 0.1 1.1 ± 0.1 2.6 ± 0.2 1.6 ± 0.1 1.4 ± 0.1 5.0 ± 0.3 3.4 ± 0.1 0.6 ± 0.0 0.2 ± 0.0 0.2 ± 0.0 0.8 ± 0.1 1.0 ± 0.1 0.9 ± 0.0 3.0 ± 0.2 0.8 ± 0.0 0.4 ± 0.0 18:0 3.7 ± 0.1 2.7 ± 0.2 4.1 ± 0.2 10.0 ± 0.3 6.2 ± 0.3 18.8 ± 0.8 6.6 ± 0.2 2.5 ± 0.1 1.2 ± 0.1 1.0 ± 0.1 5.8 ± 0.3 13.0 ± 0.5 10.1 ± 0.4 9.1 ± 0.4 8.3 ± 0.3 1.8 ± 0.1 18:1 17.9 ± 0.9 23.1 ± 1.2 15.3 ± 0.6 24.0 ± 1.4 13.6 ± 0.8 11.1 ± 0.5 18.1 ± 1.0 15.0 ± 0.4 35.2 ± 1.2 38.2 ± 1.9 25.7 ± 1.1 25.2 ± 0.9 12.8 ± 0.7 12.3 ± 0.7 14.8 ± 0.8 16.8 ± 0.7 18:2 20.8 ± 1.2 21.6 ± 0.9 13.3 ± 0.5 15.9 ± 0.6 5.3 ± 0.3 6.7 ± 0.2 22.2 ± 0.9 18.6 ± 0.9 20.2 ± 1.1 18.6 ± 0.5 19.0 ± 0.6 17.8 ± 0.8 5.8 ± 0.2 8.7 ± 0.3 5.3 ± 0.2 17.8 ± 0.5 18:3 n-6 1.4 ± 0.1 1.2 ± 0.1 1.2 ± 0.1 1.4 ± 0.1 1.2 ± 0.0 1.6 ± 0.1 0.4 ± 0.0 1.6 ±0.1 0.5 ± 0.0 0.5 ± 0.0 1.6 ± 0.1 1.6 ± 0.1 0.7 ± 0.0 2.0 ± 0.1 1.3 ± 0.1 1.0 ± 0.1 18:3 n-3 10.8 ± 0.4 8.9 ± 0.6 10.7 ± 0.7 8.4 ± 0.3 2.6 ± 0.1 9.8 ± 0.6 11.6 ± 0.4 10.2 ± 0.5 7.8 ± 0.5 7.4 ± 0.2 8.5 ± 0.3 7.1 ± 0.4 1.1 ± 0.1 14.1 ± 0.8 17.0 ± 0.6 9.1 ± 0.3 18:4 1.1 ± 0.1 1.0 ± 0.0 1.3 ± 0.1 1.2 ± 0.1 0.9 ± 0.0 3.5 ± 0.2 1.0 ± 0.1 0.9 ± 0.1 0.4 ± 0.0 0.4 ± 0.0 1.3 ± 0.1 1.7 ± 0.1 0.6 ± 0.0 3.6 ± 0.2 0.4 ± 0.0 0.5 ± 0.0 SFA (%) 30.3 ± 1.2 27.7 ± 1.0 24.3 ± 0.5 35.9 ± 1.6 35.3 ± 1.4 44.3 ± 2.5 27.2 ± 1.1 39.9 ± 2.2 23.6 ± 1.3 22.8 ± 1.0 26.0 ± 1.2 34.6 ± 1.9 60.5 ± 2.7 28.0 ± 0.8 35.7 ± 1.3 44.9 ± 2.3 MUFAc (%) 20.1 ± 0.7 25.1 ± 1.1 25.1 ± 1.6 25.7 ± 1.0 17.3 ± 0.5 14.0 ± 0.6 19.0 ± 1.0 16.0 ± 0.4 37.4 ± 1.3 41.2 ± 1.6 30.3 ± 1.5 27.5 ± 1.2 19.5 ± 1.1 14.5 ± 0.6 17.1 ± 0.5 18.3 ± 1.0 49.6 ± 2.3 47.2 ± 2.1 50.6 ± 2.7 38.4 ± 1.3 47.4 ± 1.6 41.7 ± 2.2 53.8 ± 2.7 44.1 ± 1.9 39.1 ± 2.1 36.0 ± 1.5 43.7 ± 1.7 37.9 ± 2.1 20.0 ± 1.1 57.5 ± 1.9 47.2 ± 2.4 36.8 ± 2.0 1.42 ± 0.1 1.39 ± 0.1 1.56 ± 0.1 1.23 ± 0.0 1.52 ± 0.1 1.36 ± 0.1 1.55 ± 0.1 1.24 ± 0.1 1.28 ± 0.1 1.26 ± 0.1 1.35 ± 0.1 1.20 ± 0.0 0.71 ± 0.0 1.72 ± 0.1 1.35 ± 0.1 1.05 ± 0.1 b d PUFA (%) ▽/mol e a Data are expressed as mean ± standard deviation of triplicates, cells harvested after 14 days of cultivation. TL, total lipids; TAG, triacylglycerol; DAG, diacylglycerol; MAG, monoacylglycerol; SE, steroid ester; FFA, free fatty acids; GL, glycolipids; PL, phospholipids. b SFA, saturated fatty acids; c MUFA, monounsaturated fatty acids; d PUFA, polyunsaturated fatty acid; e ▽/mol, the degree of fatty acid unsaturation = [1.0 (% monoenes) + 2.0 (% dienes) + 3.0 (% trienes) + 4.0 (% tetraenes) ]/100. 130 Chapter 6. Differential lipid and fatty acid profiles of photoautotrophic and heterotrophic Chlorella zofingiensis: assessment of algal oils for biodiesel prodoction The overall distribution of TFA and oleic acid in the lipid classes of heterotrophic cells is depicted in Figure 6.3. It was observed that heterotrophic C. zofingiensis could produce a large amount of TAG (70.5% of Total lipids) as the major storage lipid which contained 81.2% and 90.0% of TFA and total oleic acid respectively. Since intracellular lipids can be enhanced by controlling the culture conditions, such as limitation of nutrients (e.g., nitrogen, phosphorus, sulfur) (Takagi et al., 2000; Khozin-Goldberg & Cohen, 2006) and salt stress (Takagi et al., 2006), it can be expected that the TAG yields of heterotrophic C. zofingiensis cells can further be increased by the same approaches. Figure 6.3 Overall distribution of TFA (white column) and oleic acid (gray column) in various lipid classes of heterotrophic C. zofingiensis. 6.6 Discussion The cell biomass and cellular lipid content are two key factors for the initial assessment of a microalga for biodiesel production. Under photoautotrophic growth conditions, both cell biomass and lipid content of C. zofingiensis were low 131 Chapter 6. Differential lipid and fatty acid profiles of photoautotrophic and heterotrophic Chlorella zofingiensis: assessment of algal oils for biodiesel prodoction as compared with those from heterotrophic growth conditions (Table 6.1 and Figure 6.2A). Accordingly, a much higher TFA content was observed in heterotrophic cells (Figure 6.1B). The lipid yield of heterotrophic cells was 4.96 g L-1, about 9 times higher than that of photoautotrophic cells (0.49 g L-1). NL especially TAG has priority over PL or GL for biodiesel production due to their higher content of fatty acids. In this regard, oils from heterotrophic cells are more feasible for biodiesel production than that from the photoautotrophic cells since heterotrophic cells achieved TAG up to 3.49 g L-1 while photoautotrophic ones only 0.94 g L-1. The composition and structure of fatty acid esters, such as unsaturation degree and carbon chain length, determine the properties (e.g. cetane number, viscosity, cold flow, oxidative stability, and iodine value) of biodiesel (Knothe, 2005). The fatty acids from C. zofingiensis were in medium length (16 to 18 carbons) with the maximum unsaturation degree being 3 (Table 6.2), which are similar to that of plant oils currently used for biodiesel production (Singh & Singh, 2010). Compared with photoautotrophic cells, heterotrophic cells attained a much higher amount of oleic acid which better balances oxidative stability and low-temperature properties and therefore promotes the quality of biodiesel (Knothe, 2008, 2009). With regarding to the oil unsaturation, oil from photoautotrophic cells had an iodine value of 133.5 g I2/100 g that is over the European standard of biodiesel (120 g I2/10g); whereas the iodine value of oil from the heterotrophic cells (117.9 g I2/100 g) complied with the standard. The general interest of using photoautotrophic algal cells for biodiesel production is that sunlight and CO2 can be directly converted to oils. Due to the low cell density and cellular lipid content by photoautotrophic cells, many technical challenges remain such that algae have to be harvested from large, shallow ponds and oils separated from the cells. To avoid those pitfalls, sugar was employed to boost biomass and lipid yield by C. zofingiensis. The study clearly showed that heterotrophic algal cells attained a much higher yield of oils than photoautotrophic cells. Furthermore, oils from heterotrophic cells are more 132 Chapter 6. Differential lipid and fatty acid profiles of photoautotrophic and heterotrophic Chlorella zofingiensis: assessment of algal oils for biodiesel prodoction feasible for biodiesel production due to their high contents of NL and oleic acid. The major drawback of using heterotrophic cultures for oils is the need of glucose to feed the cells and in the meanwhile the emission of CO2. Glucose takes up about 80% of total medium cost and depends on food-based agriculture (Li et al., 2007). One strategy to overcome the drawback is to grow C. zofingiensis on the sugars from industrial or agricultural waste and other “cellulosic” materials (Xu et al., 2006; Jiang et al., 2009). It was found that cane molasses, a by-product of sugar industry consisting of approximate 40-50% (w/w) of total sugars (Najafpour & Poi Shan, 2003), could be ideally utilized by C. zofingiensis for lipid yield, which will be described in next chapter. 133 Chapter 7. Sugar-based growth and lipid accumulation for oil production in heterotrophic Chlorella zofingiensis Chapter 7 Sugar-based growth and lipid accumulation for oil production in heterotrophic Chlorella zofingienesis 7.1 Abstract The lipid production and fatty acid profile of Chlorella zofingiensis cultured in the dark with various carbon sources were investigated. Of the sugars surveyed, glucose was found to be the best one for the growth and lipid production. When cultivated with 50 g L-1 of glucose, C. zofingiensis accumulated lipids up to 52% of the dry weight, with triacylglycerol (TAG) accounting for 72.1% of the total lipids. Fatty acid profiles revealed that glucose contributed to the highest yield of total fatty acids (TFA) and proportion of oleic acid (35.7% of TFA), which corresponded to the strongest up-regulation of biotin carboxylase (BC) and stearoyl ACP desaturase (SAD) genes. In fed-batch cultivation based on glucose, the lipid yield and productivity of C. zofingiensis were further increased to 20.7 g L-1 and 1.38 g d-1 L-1 respectively, representing around 3.9-fold of those achieved in batch culture. Moreover, the low cost cane molasses was surveyed and proved to be an ideal carbon source for boosting lipid production by C. zofingensis. These results suggest that C. zofingiensis has great potential for biodiesel production. 134 Chapter 7. Sugar-based growth and lipid accumulation for oil production in heterotrophic Chlorella zofingiensis 7.2 Introduction Chapter 6 described the superiority of heterotrophically grown C. zofingiensis cells over photoautotrophically grown ones as biodiesel feedstocks. In this chapter, to further explore the potential and economical feasibility of heterotrophic C. zofingiensis as a biodiesel feedstock, various organic carbon sources were surveyed. Firstly, two disaccharides (lactose and sucrose) and four monosaccharides (galactose, fructose, mannose and glucose) were employed to test the effect of sugars on heterotrophic growth and lipid production of C. zofingiensis. Secondly, cane molasses was pretreated and adopted to develop a cost-effective culture medium for lipid production by C. zofingiensis in heterotrophic growth mode. Cane molasses is a byproduct of the sugar industry, consisting of approximately 50% (w/w) total sugars (sucrose, glucose and fructose), water, suspended colloids, heavy metals, vitamins and nitrogenous compounds, etc. (Najafpour & Poi Shan, 2003). It is a low-cost raw material, readily available and has been widely used to feed bacteria or yeast for the production of various industrial important chemicals (Quesada-Chanto et al., 1994; Sharma et al., 2008; Liu et al., 2009b; Jiang et al., 2009). This chapter, for the first time, reported the economical production of lipids by C. zofingiensis from pretreated cane molasses. The regulation of two key genes involved in fatty acid biosynthesis was also surveyed in order to deepen our fundamental understanding of biodiesel formation. 7.3 Methods and materials 7.3.1 Algal strain and culture conditions The maintenance and inoculation of C. zofingiensis were described in 2.3.1. 135 Chapter 7. Sugar-based growth and lipid accumulation for oil production in heterotrophic Chlorella zofingiensis 7.3.2 Pretreatment of molasses Cane molasses was obtained from Jiang-men sugar-refinery (Guangdong, PRC), and it contained 35% (w/w) sucrose, 10% (w/w) converted sugars (glucose and fructose), 2.5% (w/w) other carbohydrates, 4.3% (w/w) crude protein, 0.06% (w/w) crude fat, 9.6% (w/w) ash, 4.6% (w/w) salt, 8.9% (w/w) metal ions such as calcium, potassium, sodium, iron, magnesium, copper, etc., and 25% (w/w) water. For sulfuric acid treatment to remove cations, the molasses solution was adjusted to pH 3.5 with 5 M H2SO4, and heated at 60 °C for 2 h; after centrifugation at 10,000 g for 15 min, the supernatant was adjusted to pH 6.5 with 10 M NaOH (Jiang et al., 2009). For absorption treatment, the activated charcoal (Sigma) was added to the molasses solution and the mixture was heated with continuous stirring at 50 °C for 15 min; activated charcoal was removed by filtration and the process was repeated until the solution was colorless (Kotzamanidis et al., 2002). 7.3.3 Batch and fed-batch culture For batch culture, flasks, each containing 90 mL medium supplemented with various carbon sources (lactose, galactose, sucrose, fructose, mannose, glucose and molasses), were inoculated with 10% (v/v) of exponentially growing inoculum and then incubated at 25 °C in an orbital shaker at 150 rpm in the dark. For fed-batch culture, a two-stage fed-batch strategy was adopted using a 3.7-L fermenter (Bioengineering Ag, Wald, Switzerland), as previously described (Sun et al., 2008). The working volume of the fermentor was 3.0 L. The cultivation conditions in the fermenter were controlled as follows: pH 6.5; temperature 25 °C; agitation 450 rpm; and dissolved oxygen concentration at 50% saturation. During fed-batch cultivation, the sterilized stock nutrient solution was fed into the fermenter to maintain the sugar concentration at 5-20 g L-1. 136 Chapter 7. Sugar-based growth and lipid accumulation for oil production in heterotrophic Chlorella zofingiensis 7.3.4 Determination of glucose and nitrate concentration, cell dry weight and specific growth rate Determination of glucose and nitrate concentration, cell dry weight and specific growth rate were described in 6.3.2. 7.3.5 Lipid extraction and analysis Lipid extraction and analysis were described in 6.3.3. 7.3.6 Fatty acid analysis Fatty acid analysis was described in 6.3.4. 7.3.7 RNA isolation and RT-PCR assay RNA isolated and reverse transcription were described in 2.3.5. PCR amplification was carried out using specific primers of biotin carboxylase (BC) and stearoyl ACP-desaturase (SAD) genes (Table 7.1). C. zofingiensis actin (ACT) primers were used to demonstrate equal amounts of templates and loading. The GenBank accession numbers for BC and SAD were GQ996717 and GQ996719, respectively. Amplification was done by conventional PCR [94 °C for 2 min followed by 24 cycles (for ACT gene) or 26 cycles (for BC and SAD genes) of 94 °C for 15 s, 58 °C for 20 s, 72 °C for 30 s]. 137 Chapter 7. Sugar-based growth and lipid accumulation for oil production in heterotrophic Chlorella zofingiensis Table 7.1 Primer sets for gene expression by RT-PCR Gene BC Forward Reverse SAD Forward Reverse ACT Forward Reverse Primer (5'-3') GTGCGATTGGGTATGTGGGGGTG CGACCAGGACCAGGGCGGAAAT TCCAGGAACGTGCCACCAAG GCGCCCTGTCTTGCCCTCATG TGCCGAGCGTGAAATTGTGAG CGTGAATGCCAGCAGCCTCCA 7.4 Results 7.4.1 Heterotrophic growth and lipid production of C. zofingiensis with various carbon sources The lipid and fatty acid profiles of heterotrophic C. zofingiensis were reported in chapter 6. To investigate the influences of different sugars on growth and lipid production of C. zofingiensis, the algal cells were cultured in the dark with lactose, galactose, sucrose, fructose, mannose or glucose as the carbon source, respectively. Among the tested sugars, glucose gave the highest growth rate (0.03 h-1), cell biomass (10.1 g L-1), lipid content (0.52 g g-1), and lipid yield (5.27 g L-1). Algal cells cultured with mannose, fructose, or sucrose produced slightly lower amounts of cell biomass and lipids. In contrast, lactose and galactose were observed to be poor carbon sources for lipid production since low biomass and lipid contents were obtained (Figure 7.1). These findings demonstrated a close relationship between lipid biosynthesis and cell growth for the heterotrophic alga. 138 Chapter 7. Sugar-based growth and lipid accumulation for oil production in heterotrophic Chlorella zofingiensis Glc Figure 7.1 Cell biomass (black column), lipid content (gray column) and lipid yield (white column) of C. zofingiensis cultured with 50 g L-1 various sugars. Lac, lactose; Gal, galactose; Suc, sucrose; Fru, fructose; Man, mannose; Glc, glucose. As shown in Figure 7.2, based on lipid class analysis of C. zofingiensis cultured with glucose, neutral lipids (NL) were found to be the major constituent that accounted for 80.9% of the total lipids, while phospholipids (PL) and glycolipids (GL) together accounted for 19.1%. In NL, TAG were the predominant component, accounting for 72.1% of the total lipids. In the batch culture, up to 0.375 g g-1 TAG were accumulated in heterotrophic C. zofingiensis cells. Unlike PL and GL that are membrane-bounded lipids, TAG serve primarily as a storage form of carbon and energy (Hu et al., 2008). In addition, TAG are superior to phospholipids (PL) or glycolipids (GL) for biodiesel due to their higher content of fatty acids (Pruvost et al., 2009). Hence C. zofingiensis may act as a promising host for biodiesel production due to its fast cell growth and high content of TAG. 139 Chapter 7. Sugar-based growth and lipid accumulation for oil production in heterotrophic Chlorella zofingiensis Figure 7.2 Distribution of NL, GL and PL in total lipids extracted from heterotrophic C. zofingiensis cells cultured with 50 g L-1 of glucose for 14 days. The horizontal line inside the NL bar marks the portion of TAG in this fraction. 7.4.2 Fatty acid profiles of dark-grown C. zofingiensis cultures Fatty acid composition considerably influences the properties of biodiesel such as cetane number, heat of combustion, oxidative stability, cloud point, lubricity, which finally influences the quality of biodiesel (Knothe, 2009). Here the fatty acid compositions of the heterotrophically cultured C. zofingiensis with various sugars were investigated (Table 7.2). It was found that C16:0, C16:2, C18:1, C18:2 and C18:3 (n-3) were the major fatty acids, which together accounted for more than 84% of the total fatty acids (TFA). The highest amounts of TFA (45.4% of dry weight) and oleic acid (35.7% of TFA) were achieved in C. zofingiensis cultured with glucose as the carbon source, which were about 5 and 3 times respectively of those given by lactose. As an ideal biodiesel, the fatty acid esters should be oxidative and low-temperature stable. Generally saturated fatty acid esters are oxidative stable, while unsaturated fatty acid esters give low-temperature stability (Knothe, 2008). The enhanced proportion of oleic acid ester in the total fatty acid esters has been 140 Chapter 7. Sugar-based growth and lipid accumulation for oil production in heterotrophic Chlorella zofingiensis considered as a feasible approach to balance the oxidative and low-temperature stability with retaining the cetane number at an acceptable level (Knothe, 2009). The high content of oleic acid in the heterotrophic cells further supports that C. zofingiensis is a favorable host for producing high quality biodiesel. Table 7.2 Fatty acid profiles of dark-grown C. zofingiensis cultured with 50 g L-1 various sugars for 14 days a Fatty acids Sugars Lactose Galactose Sucrose Fructose Mannose Glucose C16:0 28.73 ± 1.22 27.81 ± 0.99 23.16 ± 0.83 23.62 ± 1.12 23.38 ± 1.03 22.62 ± 0.77 C16:1 3.42 ± 0.12 2.52 ± 0.13 1.49 ± 0.06 1.71 ± 0.08 1.49 ± 0.05 1.97 ± 0.08 C16:2 9.02 ± 0.35 11.08 ± 0.61 8.48 ± 0.39 8.31 ± 0.44 7.94 ± 0.29 7.38 ± 0.33 C16:3 5.28 ± 0.27 2.52 ± 0.11 1.96 ± 0.07 2.12 ± 0.09 1.83 ±0.06 1.94 ± 0.04 C16:4 0.92 ± 0.05 0.65 ± 0.03 0.23 ± 0.02 0.17 ± 0.01 0.19 ± 0.01 0.22 ± 0.02 C18:0 0.95 ± 0.06 1.61 ± 0.09 2.80 ± 0.09 2.34 ± 0.12 2.44 ± 0.10 2.09 ± 0.08 C18:1 12.59 ± 0.45 25.15 ± 0.89 31.99 ± 1.21 32.06 ± 0.93 33.30 ± 1.45 35.68 ± 1.23 C18:2 20.87 ± 0.87 16.48 ± 0.83 19.51 ± 0.75 19.27 ± 0.49 19.36 ± 0.98 18.46 ± 0.66 C18:3 (n-6) 1.39 ± 0.03 1.17 ± 0.05 0.55 ± 0.01 0.52 ± 0.03 0.52 ± 0.03 0.51 ± 0.02 C18:3 (n-3) 12.86 ± 0.39 7.74 ± 0.31 7.36 ± 0.35 7.48 ± 0.18 7.31 ± 0.23 7.24 ± 0.30 C18:4 1.24 ± 0.04 1.10 ± 0.06 0.46 ± 0.01 0.45 ± 0.01 0.45 ± 0.02 0.49 ± 0.02 Others 2.72 ± 0.09 2.17 ± 0.11 2.00 ± 0.07 1.94 ± 0.04 1.78 ± 0.08 1.40 ± 0.08 MUFAb (%) 16.01 ± 0.55 27.67 ± 0.82 33.48 ± 1.12 33.78 ± 1.03 34.79 ± 1.37 37.64 ± 1.26 PUFAc (%) 51.59 ± 2.11 40.74 ± 2.05 38.57 ± 1.28 38.33 ± 1.49 37.61 ± 1.44 36.24 ± 1.63 UFAd (%) 67.59 ± 2.95 68.42 ± 2.31 72.05 ± 3.72 72.10 ± 3.11 72.40 ± 3.13 73.89 ± 2.99 ▽/mol e 1.43 ± 0.05 1.24 ± 0.06 1.22 ± 0.06 1.22 ± 0.03 1.21 ± 0.03 1.21 ± 0.05 TFA f 8.48 ± 0.33 20.30 ± 0.71 40.48 ± 1.29 43.01 ± 1.11 43.10 ± 1.79 45.38 ± 1.83 a Data are expressed as mean ± standard deviation of triplicates. b MUFA, monounsaturated fatty acids; c PUFA, polyunsaturated fatty acids; d UFA, unsaturated fatty acids; e ▽/mol, degree of fatty acid unsaturation = [1.0 (% monoenes) + 2.0 (% dienes) + 3.0 (% trienes) + 4.0 (% tetraenes) ]/100; f TFA, total fatty acids (g) / cell dry weight (g) × 100%. 141 Chapter 7. Sugar-based growth and lipid accumulation for oil production in heterotrophic Chlorella zofingiensis 7.4.3 Sugars up-regulate the transcription of BC and SAD genes of C. zofingiensis The de novo biosynthesis of fatty acid is initially catalyzed in chloroplast by the key enzyme acetyl-CoA carboxylase (ACCase); while the introduction of the first double bond to acyl chain is carried out by stearoyl ACP (SAD), the enzyme playing an important role in determining the ratio of unsaturated and saturated fatty acids (Hu et al., 2008). To reveal the relationship between the fatty acid profiles and the regulation of ACCase and SAD, we investigated the transcript levels of SAD and biotin carboxylase (BC, a subunit of ACCase) in dark-grown C. zofingiensis cells cultured with various sugars using the RT-PCR approach. The transcription of the two genes was shown to be differentially regulated by various carbon sources (Figure 7.3). In coincidence with the higher contents of TFA in the algal cells cultured with sucrose, fructose, mannose or glucose, the transcript levels of BC in the cells were strongly up-regulated. In contrast, galactose moderately enhanced the transcription of BC whereas lactose, the poor carbon source for the cell growth and fatty acid accumulation, caused a slight up-regulation of BC. These results are consistent with the findings of Ohlrogge and Jaworski (1997) that the first reaction step of fatty acid biosynthesis catalyzed by ACCase is a major point of flux control for this pathway. A similar pattern induced by sugars was also found for SAD at mRNA levels, although much stronger up-regulation was observed when compared with the expression of BC (Figure 7.3). Similarly, lactose induced low-level expression of SAD while glucose triggered high amounts of SAD transcripts, which is consistent with the unsaturation values of fatty acids in the algal cells cultured with the tested sugars (Table 7.2). 142 Chapter 7. Sugar-based growth and lipid accumulation for oil production in heterotrophic Chlorella zofingiensis Figure 7.3 Transcript levels biotin carboxylase (BC) and stearoyl ACP desaturae (SAD) genes in heterotrophic C. zofingiensis cultured with 50 g L-1 of various sugars. The expression of actin (ACT) gene was used as control. Ctr, control cultured without sugar. Abbreviations see Figure 7.1. 7.4.4 Fed-batch fermentation enhances lipid production by C. zofingiensis As shown by the above results, the productivity of lipid is closely related to the growth rate, cell density and cellular lipid content. Compared with batch culture, fed-batch cultivation mode can extend the exponential growth phase of the cell and further increase the biomass concentration fed with key medium components or fresh medium at time intervals. It was indicated that C. zofingiensis could reach a high specific growth rate (ca. 0.03 h-1) and a high cell-density (ca. 53 g L-1) in a fed-batch culture system (Sun et al., 2008). To further evaluate the lipid production of C. zofingiensis, a 3.7-L fermenter was used to investigate the effect of glucose on cell growth and lipid accumulation in a fed-batch culture system. The fed-batch procedure contained two stages of feeding: three times of feeding with glucose-containing medium and four times of feeding with glucose. The feeding time, time course of cell growth and lipid production are shown in Figure 7.4. In the fed-batch fermentation a high lipid yield of 20.7 g L-1 was obtained, with a lipid productivity of 1.38 g d-1 L-1. At the stage-fed with glucose-containing medium, the algal cells grew fast but the cellular lipid content 143 Chapter 7. Sugar-based growth and lipid accumulation for oil production in heterotrophic Chlorella zofingiensis was low (data not shown), possibly due to the relatively low carbon/nitrogen (C/N) ratio as indicated by the remaining concentrations of glucose and nitrate in the medium (Figure 7.4). When stage-fed with glucose, due to the consumption of nitrogen and the new addition of glucose, a high C/N ratio formed in the culture medium, resulting in a favorable condition for lipid accumulation within cells (Figure 7.4). These results further support that C. zofingiensis has the potential to be cultivated by using fermentation biotechnology for large-scale production of lipids. Figure 7.4 Cell biomass, glucose and nitrate consumption and lipid production in a two-stage fed-batch fermentation of C. zofingiensis in a 3.7-L fermenter. (■) glucose concentration; (○) cell biomass; (column) lipid content; (□) lipid yield; (◇) NO3- concentration; (↓) glucose-containing medium feeding; (↓↓) glucose feeding. 144 Chapter 7. Sugar-based growth and lipid accumulation for oil production in heterotrophic Chlorella zofingiensis 7.4.5 Assessment of cane molasses as the carbon source for lipid production by C. zofingiensis C. zofingiensis could efficiently utilize glucose, fructose and sucrose for growth and lipid production (Figure 7.1). Thus, it is reasonable to expect that the low-cost cane molasses can be used to feed C. zofingiensis for economical production of algal oils. However, the untreated molasses gave relatively low cell biomass (5.5 g L-1) and lipid content (0.32 g g-1) and thus the low lipid yield (1.76 g L-1) (Table 7.3). Molasses normally contains most of the essential nutrients for the growth of microorganisms, but it also contains lots of metal ions and suspended colloids, which could inhibit the growth of microorganisms and contribute to the inactivation of certain enzymes associated with product biosynthesis (Jiang et al., 2009). The pretreatment of cane molasses is therefore necessary. Sulfuric acid treatment is regarded as an efficient way to remove cations and has been widely used for molasses pretreatment (Roukas et al., 1998; Kotzamanidis et al., 2002; Kalogiannis et al., 2003; Jiang et al., 2009). Compared with the untreated molasses, the sulfuric acid treated one induced the significantly higher cell biomass (10.8 g L-1) and lipid content (0.48 g g-1) and thus the much greater lipid yield (5.18 g L-1) (Table 7.3). The absorption treatment using charcoal to remove color was also tested, but proved to be less efficient than the sulfuric acid treatment, as indicated by the lower lipid yield (Table 7.3). In addition, the combination of the above two treatments was not as good as sulfuric acid treatment alone for producing lipids by C. zofingiensis (Table 7.3). The fed-batch fermentation of C zofingiensis using the sulfuric acid treated molasses for lipid production was also examined. Compared with glucose, the pretreated molasses gave a slightly higher cell biomass but lower cellular lipid content (Table 7.4). This might be explained by that molasses contains many essential nutrients and exerts positive effect on the growth of this green alga. The lipid yield and sugar-based lipid yield coefficient obtained from C. zofingiensis 145 Chapter 7. Sugar-based growth and lipid accumulation for oil production in heterotrophic Chlorella zofingiensis cultured with the pretreated molasses were 19.7 g L-1 and 0.239 respectively, which are comparable to those obtained from the alga cultured with glucose (Table 7.4). These results indicate that cane molasses, when pretreated with proper methods, can substitute glucose to feed C. zofingiensis for economical production of heterotrophic algal oils. Table 7.3 Lipid production from pretreated molasses by C. zofingiensis in batch culture systems a Pretreatment of Lipid yield (g L-1) Residual total Sugar molasses Cell biomass Lipid content (g L-1) (g g-1) sugar (g L-1) unitization (%) Untreated 5.5 ± 0.29 0.32 ± 0.02 1.76 ± 0.10 15.8 ± 0.65 47.4 ± 2.5 Sulfuric acid treatment 10.8 ± 0.43 0.48 ± 0.03 5.18 ± 0.22 4.6 ± 0.28 84.7 ± 3.3 Absorption treatment 7.9 ± 0.28 0.39 ± 0.01 3.08 ± 0.17 11.7 ± 0.46 61.0 ± 3.5 Sulfuric acid + absorption 9.1 ± 0.47 0.43 ± 0.02 3.91 ± 0.21 6.8 ± 0.39 77.3 ± 4.2 a Data are expressed as mean ± standard deviation of triplicates. Table 7.4 Lipid production from pretreated molasses by C. zofingiensis in fed-batch fermenter systems a Sugar Cell biomass Lipid content (g L-1) (g g-1) Lipid yield (g L-1) Consumed sugar (g L-1) Lipid yield coefficient b Glucose 42.5 ± 1.9 0.48 ± 0.02 20.7 ± 0.8 83.9 ± 4.5 0.243 ± 0.01 Pretreated molasses 46.9 ± 2.3 0.42 ± 0.02 19.7 ± 1.2 82.3 ± 3.1 0.239 ± 0.02 a Data are expressed as mean ± standard deviation of triplicates. b Lipid yield coefficient = lipid yield (g L-1) / consumed sugar (g L-1). 7.5 Discussion C. zofingiensis can grow heterotrophically using sugars as the carbon and energy sources (Ip & Chen, 2005; Sun et al., 2008). In the current chapter, several 146 Chapter 7. Sugar-based growth and lipid accumulation for oil production in heterotrophic Chlorella zofingiensis sugars were tested for lipid production by heterotrophic C. zofingiensis cells. Glucose, mannose, fructose and sucrose were shown to be suitable organic carbon sources for boosting lipid yield (Figure 7.1). Glucose also induced a high proportion of NL including TAG (Figure 7.2), which are regarded to be superior to GL and PL when considered as biodiesel sources. Fatty acid profile is also the important information for the assessment of biodiesel feedstocks. As shown in Table 7.2, glucose-fed algal cells were lower in saturated fatty acids and higher in oleic acid, which are considered as good for the balance between oxidative stability and cold flow of oils and thus be able to promote the quality of biodiesel (Knothe, 2009). What’s more, the high lipid yield (20.7 g L-1) and lipid productivity (1.38 g d-1 L-1) were obtained by using a well designed fed-batch fermentation strategy (Figure 7.4). Compared with other microalgae, C. zofingiensis showed obvious advantages in terms of lipid content and lipid productivity (Table 5). All these results together implicate that the heterotrophic C. zofingiensis might have the potential to be a biodiesel feedstock. Table 7.5 Lipid production by C. zofingiensis and other microalgae Microalgal species Lipid content (% dry weight) Lipid yield (mg L-1 day-1) References C. zofingiensis 48.0 1380 This study Chlorella protothecoides 14.6-57.8 1214 Chlorella emersonii 25.0-63.0 10.3-50.0 Chlorella sorokiniana 19.0-22.0 44.7 Chlorella vulgaris 5.0-58.0 11.2-40.0 Mata et al., Chlorococcum sp. 19.3 53.7 2010 Dunaliella salina 6.0-25.0 116.0 Nannochloropsis oculata 22.7-29.7 84.0-142.0 Neochloris oleoabundans 29.0-65.0 90.0-134.0 147 Chapter 7. Sugar-based growth and lipid accumulation for oil production in heterotrophic Chlorella zofingiensis Other microorganisms that grow only heterotrophically such as bacteria and yeasts have also been explored for biodiesel sources. Bacteria are less used for biodiesel production since they have low contents of oils and produce mostly complex lipid (Kalscheuer et al., 2006; Gouda et al., 2008). Yeasts have the ability of accumulating high levels of cellular lipids, ranging from 5.32 to 37.1 g L-1 (Li et al., 2008c). Generally speaking, fermentation-based biodiesel production contributes the emission of CO2, which might limit the application of the microorganisms for biodiesel production to some extent. Unlike yeasts, C. zofingiensis can grow well both photoautotrophically, mixotrophically and heterotrophically. Thus, by switching its growth modes, C. zofingiensis may release much less CO2 during the accumulation of lipids, as has been demonstrated by Xiong et al. (2010). The major drawback of using heterotrophic cultures for oils is the need of glucose to feed the cells because glucose, which takes up about 60% of total cost, depends on food-based agriculture. The current price of industrial glucose is around 340 USD per ton. According to Table 7.4, the conversion ration of sugar to lipid is about 24.3%. Thus the roughly estimated cost of one kilogram oil obtained from C. zofingiensis fed with glucose is around $2.33, 3-4 times higher than plant oil. One strategy to overcome the drawback is to grow C. zofingiensis on the sugars from industrial or agricultural waste and other “cellulosic” materials (Xu et al., 2006; Jiang et al., 2009). Cane molasses, a low-cost by-product of sugar industry containing approximate 40-50% (w/w) of total sugars, were proved to be an ideal carbon source utilized by C. zofingiensis for lipid production (Table 7.3 and 7.4). Currently, the price of cane molasses is around one fifth of glucose, which means the cost of microalgal oil can be reduced to $1.52 per kilogram when molasses replaces glucose as the sugar source. Moreover, C. zofingiensis could yield high amount of the high-value astaxanthin under heterotrophic conditions (Ip & Chen, 2005). Thus, by feeding algae with “waste” sugars and tying algal oil production to other products (e.g., astaxanthin), profitable biodiesel from microalgae won’t remain a research project for long. 148 Chapter 8. Production of fatty acids by heterotrophic Chlorella zofingiensis: influences of nutritional and environmental factors Chapter 8 Production of fatty acids by heterotrophic Chlorella zofingiensis: influences of nutritional and environmental factors 8.1 Abstract Biodiesel is chemically referred to as fatty acid methyl esters. This chapter surveyed the effect of several nutrients, namely, nitrate, carbon/nitrogen (C/N) ratio, phosphate and ferrous ion as well as the environmental conditions including temperature and the initial pH of culture medium on the growth and fatty acid profile of heterotrophic C. zofingiensis in batch culture. Results showed that cell growth was promoted at sufficient nitrogen while the accumulation of total fatty acids (TFA) was enhanced under nitrogen starvation/deprivation. A high C/N ratio (e.g., 200) was found to be beneficial for the accumulation of TFA including oleic acid (C18:1). Similar to nitrogen, phosphorus favored cell growth at sufficient concentrations but induced TFA accumulation at starved/deprived concentrations. Ferrous ion was required in a low concentration (25 μM) to maximize cell growth and TFA accumulation. The alga grew well at a wide range of temperatures (25-30 °C) and initial pH (4.5-8.5) of the culture medium for TFA accumulation. The optimal nutritional and environmental conditions for TFA production by C. zofingiensis were 5 mM nitrate, 5 mM phosphate, 25-100 μM ferrous ion, cultivation temperature of 25 °C and the initial pH of 6.5. The results of this study may serve as a guide to a large scale cultivation of C. zofingiensis and possible other algae for biodiesel. 149 Chapter 8. Production of fatty acids by heterotrophic Chlorella zofingiensis: influences of nutritional and environmental factors 8.2 Introduction Biodiesel, also known as fatty acid methyl esters, is derived from the transesterification of feedstocks. Thus the yield and quality of biodiesel from a feedstock are largely determined by the TFA content and fatty acid properties of the feedstock. Microalgae have been considered as the most potential feedstocks for biodiesel production (Chisti, 2007; Huang et al., 2010; Mata et al., 2010). The fatty acid profiles of microalgae are species/strain-specific and can be greatly affected by the culture conditions, e.g., nutritional factors and environmental conditions, as reviewed in 1.3.5.2. Chapter 6 and 7 described the potential of heterotrophic C. zofingiensis as a biodiesel feedstock. To further explore this potential, in this study, the effect of several important factors including nitrate, phosphate, ferrous ion, cultivation temperature and the initial pH of medium on the growth and fatty acid profile of heterotrophic C. zofingiensis in batch culture were surveyed. 8.3 Methods and materials 8.3.1 Algal strain and culture conditions The maintenance of C. zofingiensis was described in 2.3.1. In the experiments, the alga was grown in the medium supplemented with 30 g L-1 glucose unless otherwise stated. To investigate the effect of nutrient concentration, nitrate and phosphate were tested at 0-10 mM; ferrous ion was used at 0-100 μM. To examine the effect of C/N ratio, glucose was fixed at three different concentrations of 10 g L-1, 30 g L-1 and 50 g L-1, with four different C/N ratios of 50, 100, 200 and 400 respectively. To survey the effect of environments, cultivation temperatures (22-35 °C) and the initial pHs (4.5-8.5) were employed. Unless otherwise stated, the media were adjusted to pH 6.5 prior to autoclaving at 150 Chapter 8. Production of fatty acids by heterotrophic Chlorella zofingiensis: influences of nutritional and environmental factors 121 °C for 20 min. An inoculum of 10% (by volume, average cell concentration of 0.5 g L-1 dry weight) was inoculated into 250-ml flasks each containing 100 ml medium under dim light. The cultures were then incubated with orbital shaking at 150 rpm in the dark for 14 days. 8.3.2 Determination of cell dry weight The determination of cell dry weight was described in 6.3.2. 8.3.3 Fatty acid analysis Fatty acid analysis was described in 6.3.4. 8.3.4 Statistical analysis Statistical analyses of TFA yield were performed using the SPSS statistical package. One way ANOVA test was applied. The statistical significances were achieved when P < 0.05. 8.4 Results and discussion 8.4.1 Nitrate Nitrogen is one of the most critical nutrients that affect cell growth and lipid/fatty acid metabolism of microalgae. The heterotrophic growth and fatty acid profile of C. zofingiensis at various nitrate concentrations were analyzed and shown in Figure 8.1. The algal cell growth was retarded at low nitrate concentrations (0-1.25 mM) and got promoted at high nitrate concentrations (5-10 151 Chapter 8. Production of fatty acids by heterotrophic Chlorella zofingiensis: influences of nutritional and environmental factors mM) (Figure 8.1A). In contrast, the cellular TFA content was enhanced at low nitrate concentrations (Figure 8.1A), which is consistent with previous studies showing that nitrogen starvation/deficiency favored the accumulation of lipids and TFA (Takagi et al., 2000; Li et al., 2005; Li et al., 2008a; Solovchenko et al., 2008; Pruvost et al., 2009). One way ANOVA test showed that the effect of nitration concentration on TFA yield was significant. The highest TFA yield was obtained at 5 mM nitrate (Figure 8.1A). Nitrate concentration also affected the algal fatty acid composition. As shown in Figure 8.1B, low nitrate concentrations enhanced the proportion of monounsaturated fatty acids (MUFA) including oleic acid (C18:1) but reduced the proportion of polyunsaturated fatty acids (PUFA). Figure 8.1 Effect of nitrate concentration on growth and fatty acid profile of heterotrophic C. zofingiensis. 152 Chapter 8. Production of fatty acids by heterotrophic Chlorella zofingiensis: influences of nutritional and environmental factors The effect of nitrate with different glucose concentrations (10, 30 and 50 g L-1) was also investigated. The C/N ratios were fixed at 50, 100, 200 and 400. Under all glucose concentrations surveyed, high C/N ratios benefited the accumulation of TFA (Figure 8.2A, B and C, left) and increased the proportion of MUFA in particular oleic acid (Figure 8.2A, B and C, right). A C/N ratio of 200 was found to enhance TFA content (Figure 8.2) and maintain cell biomass (data not shown) Figure 8.2 Effect of initial C/N ratio on TFA content (right) and fatty acid composition (left) of heterotrophic C. zofingiensis. Glucose concentrations were fixed at 10 g L-1 (A), 30 g L-1 (B) and 50 g L-1 (C). 153 Chapter 8. Production of fatty acids by heterotrophic Chlorella zofingiensis: influences of nutritional and environmental factors 8.4.2 Phosphate Figure 8.3 illustrates the effect of phosphate concentrations on the cell growth and fatty acid profile of heterotrophic C. zofingiensis. Low concentrations of phosphate restricted cell growth but promoted cellular TFA content (Figure 8.3A). Unlike nitrate, however, phosphate deprivation did not greatly inhibit the cell growth (Figure 8.3B). One way ANOVA test showed that the effect of phosphate concentration on TFA yield was not significant The TFA yield reached its maximum at 5 mM phosphate. Consistent with previous studies (Reitan et al., 1994), phosphate sufficiency gave rise to the decreased proportion of MUFA including oleic acid but the increased PUFA proportion in C. zofingiensis (Figure 8.3B). Figure 8.3 Effect of phosphate concentration on growth and fatty acid profile of heterotrophic C. zofingiensis. 154 Chapter 8. Production of fatty acids by heterotrophic Chlorella zofingiensis: influences of nutritional and environmental factors 8.4.3 Ferrous ion A significant limitation on the cell growth and TFA content of C. zofingiensis was occurred in the absence of iron (Figure 8.4A). Supplementation of ferrous ion at 25-100 μM markedly promoted the cell growth and cellular TFA content (Figure 8.4A). Similar results were also observed for the green alga Chlorella vulgaris (Liu et al., 2008). One way ANOVA test showed that the effect of ferrious concentration on TFA yield was significant. The increased concentration of ferrous ion resulted in the increased proportion of MUFA including oleic acid and decreased PUFA proportion, but almost unchanged proportion of saturated fatty acids (SFA) (Figure 4B). Figure 8.4 Effect of ferrous ion concentration on growth and fatty acid profile of heterotrophic C. zofingiensis. 155 Chapter 8. Production of fatty acids by heterotrophic Chlorella zofingiensis: influences of nutritional and environmental factors 8.4.4 Cultivation temperature C. zofingiensis grew well at the temperature of 22-28 °C; higher temperature (e.g., 32 and 35 °C) dramatically reduced the cell biomass and cellular TFA content (Figure 8.5A). Of the temperature range surveyed, 25 °C was the best temperature for the cell growth and TFA accumulation and gave rise to the highest TFA yield (Figure 8.5A). One way ANOVA test showed that the effect of cultivation temperature on TFA yield was significant. The effect of temperature on fatty acid composition was also investigated and shown in Figure 8.5B. Higher temperatures (28-35 °C) caused the greater proportion of SFA and the lower proportion of MUFA including oleic acid. This is consistent with the previous studies suggesting that increased temperature resulted in increased fatty acid saturation and the concurrently decreased fatty acid unsaturation (Wada & Murata, 1990; Thompson et al., 1992; Renaud et al., 2002; Jang et al., 2005). Figure 8.5 Effect of cultivation temperature on growth and fatty acid profile of heterotrophic C. zofingiensis. 156 Chapter 8. Production of fatty acids by heterotrophic Chlorella zofingiensis: influences of nutritional and environmental factors 8.4.5 Initial pH of culture medium Figure 8.6 shows the cell growth and fatty acid profile of C. zofingiensis in the batch culture with various initial pHs. The alga grew well at the entire range of pH surveyed. The highest cell biomass and cellular TFA content were obtained at pH 6.5, so was the TFA yield (Figure 8.6A). One way ANOVA test showed that the effect of nitration concentration on TFA yield was not significant. pH at the range of 5.5-6.5 gave rise to a slight increase in the proportion of MUFA including oleic acid and a slight decrease in PUFA proportion (Figure 8.6B). Figure 8.6 Effect of culture medium pH on growth and fatty acid profile of heterotrophic C. zofingiensis. 157 Chapter 8. Production of fatty acids by heterotrophic Chlorella zofingiensis: influences of nutritional and environmental factors In conclusion, the effect of several key factors including nitrate, C/N ratio, phosphate, ferrous ion, cultivation temperature and the culture medium pH on the cell growth and fatty acid profile of heterotrophic C. zofingiensis in the batch culture was investigated in this study. The optimal nutritional and environmental conditions for heterotrophic C. zofingiensis producing TFA were 5 mM nitrate, 5 mM phosphate, 25-100 μM ferrous ion, cultivation temperature of 25 °C and the initial pH of 6.5. 158 Chapter 9. Isolation and characterization of biotin carboxylase gene and stearoyl ACP desaturase gene from Chlorella zofingiensis Chapter 9 Isolation and characterization of biotin carboxylase gene and stearoyl ACP desaturase gene from Chlorella zofingiensis 9.1 Abstract Chlorella zofingiensis could accumulate abundant oil within cells. In order to elucidate the molecular regulation of fatty acid accumulation, the key genes involved in fatty acid biosynthesis need to be characterized. In the present study, two key genes, namely biotin carboxylase (BC) and stearoyl ACP desaturase (SAD) were cloned and characterized from C. zofingiensis. Protein sequence alignments showed that the deduced amino acid sequences of these two genes shared high identity to their corresponding genes from other microalgae and plants. High light illumination and glucose were revealed to up-regulate the expression of both BC and SAD genes, and therefore enhanced the accumulation of total fatty acids (TFA) including oleic acid (C18:1). Unlike high light or glucose, salt stress, however, could only trigger the up-regulation of SAD gene; accordingly, the TFA content was slightly affected while the biosynthesis of oleic acid was promoted. In this context, the fatty acid biosynthesis of C. zofingiensis might be regulated, at least partly, at the transcriptional level. These results deepen our understanding of fatty acid biosynthesis in C. zofingiensis, which can facilitate the manipulation of the alga for enhanced production of oils. 159 Chapter 9. Isolation and characterization of biotin carboxylase gene and stearoyl ACP desaturase gene from Chlorella zofingiensis 9.2 Introduction Similar to plants, microalgae synthesize fatty acids in the chloroplast using a single set of enzymes, of which acetyl-CoA carboxylase (ACCase) is the key one in regulating fatty acid synthesis rate while stearoyl ACP desaturase (SAD) adds the first double bond to acyl chain and plays an important role in determining the ratio of unsaturated fatty acids to saturated ones (Ohlrogge & Jaworski, 1997). Although the fatty acid composition has been shown to be regulated by the expression of its biosynthesis genes in plants, little is known with microalgae in this regard (Hu et al., 2008). To elucidate the regulation of fatty acid accumulation in microalgae, key genes need to be firstly cloned. The production potential of biodiesel using the green microalga C. zofingiensis as a feedstock was investigated in chapter 6 and 7. C. zofingiensis could produce fatty acids up to 45% of cell dry weight, with 36% of TFA being oleic acid. In the present study, two important genes encoding biotin carboxylase (BC, one subunit of ACCase) and SAD were cloned and characterized from C. zofingiensis, with the aim of examining their expressions during fatty acid biosynthesis in response to high light, salt stress or glucose. These results expand our knowledge about the fatty acid biosynthesis in C. zofingiensis and benefit enhanced fatty acid production or modified fatty acid composition through genetic engineering for better biodiesel production. 9.3 Methods and materials 9.3.1 Algal strain and culture conditions The maintenance of C. zofingiensis was described in 2.3.1. To investigate the expression of biotin carboxylase (BC) and stearoyl ACP-desaturase (SAD) genes and the biosynthesis of fatty acids under different conditions, the algal cell 160 Chapter 9. Isolation and characterization of biotin carboxylase gene and stearoyl ACP desaturase gene from Chlorella zofingiensis cultures were exposed to continuous illumination of high light (250 µmol photon m-2 s-1), maintained in the dark treated with 100 mM NaCl or 50 mM glucose for further periods. 9.3.2 Genomic DNA and RNA isolation Genomic DNA and RNA isolation were described in 2.3.2. 9.3.3 Cloning of BC cDNA, SAD cDNA and their corresponding genes The primer sets used in this study are listed in Table 9.1. To amplify the BC cDNA from C. zofingiensis, degenerate primers BC-dF and BC-dR were designed according to the conserved motifs GGGGRGM and FYFMEMNT in BC proteins from Chlamydomonas reinhardtii, Arabidopsis thaliana, Nicotiana tabacum and Brassica napus. First strand cDNA synthesis was carried out with 1 μg of total RNA extracted from 24 h high light induced cells, by using a SuperScript III First-Strand Synthesis System according to manufacturer’s instruction (Invitrogen). PCR amplification was programmed with 50 ng of cDNA as template (30 cycles of 94 °C for 30 s, 55 °C for 20 s, 72 °C for 1 min). PCR product was gel purified and sequenced, based on which specific primers (BC-F1 and BC-R2 for first round PCR, BC-F2 and BC-R2 for second round PCR) were designed for rapid amplification of 5' and 3' cDNA ends (RACE). RACE was performed by using the method described by Huang and Chen (2006). The primer pair BC-F3 and BC-R3 derived from the sequences of 5' and 3' RACE fragments were employed to amplify a full-length BC cDNA and its corresponding gene. Similar procedures were followed for SAD cDNA amplification. The degenerated primers SAD-dF and SAD-dR were derived from the conserved 161 Chapter 9. Isolation and characterization of biotin carboxylase gene and stearoyl ACP desaturase gene from Chlorella zofingiensis amino acid sequences (GDM/LITEEA and HGNTARQ/HA, respectively) of the SAD proteins from A. thaliana, B. napus, Helianthus annuus, C. reinhardtii and Haematococcus pluvialis. The primers SAD-F1, SAD-R1, SAD-F2 and SAD-R2 were used for 5' and 3' SAD RACE, while SAD-F3 and SAD-R3 were employed for amplification of the full-length SAD cDNA and its corresponding gene. Table1 Primers for gene cloning and expression Aim Oligonucleotide sequence 5'-3' Partial BC fragment BC-dF GGCGGCGGCGGNMGNGGNATG BC-dR GTRTTCATYTCCATRAARTARAA 5' and 3' BC RACE BC-F1 CGCCCTCACCCGCCCTTACAC BC-R1 CCACATTGCCATACTTGTCAGC BC-F2 GTGCGATTGGGTATGTGGGGGTG BC-R2 CCATACTTGTCAGCCAGCACC BC gene BC-F3 CCTCTGCCTACATGCCCAGCTG BC-R3 CTCAGCTGGTGCCTCATGCC BC expression BC-F2 & BC-R4 see BC-F2, CGACCAGGACCAGGGCGGAAAT Partial SAD fragment SAD-dF GGCGAYWTGATHACNGARGARGC SAD-dR GCNTGNCKNGCNGTRTTNCCRTG 5' and 3' SAD RACE SAD-F1 GGGTTTCATCTACACCTCCTTC SAD-R1 TGTTCTCCTCAGCCGTCCACTCAC SAD-F2 TCCAGGAACGTGCCACCAAG SAD-R2 CACGCGTCCACCTGCCCCAAG SAD gene SAD-F3 CTCCTACTTTGGCACACTCAGC SAD-R3 GCTTTCAATCTAGCTACTGTCG SAD expression SAD-F2 & SAD-R4 see SAD-F2, GCGCCCTGTCTTGCCCTCATG 162 Chapter 9. Isolation and characterization of biotin carboxylase gene and stearoyl ACP desaturase gene from Chlorella zofingiensis 9.3.4 RT-PCR assay RT-PCR assay of BC, SAD and actin genes was described in 7.3.7. 9.3.5 Fatty acid analysis Fatty acid analysis was described in 6.3.4. 9.4 Results 9.4.1 Cloning and characterization of the BC and SAD gene from C. zofingiensis A 380 bp fragment of BC cDNA was amplified by RT-PCR with the degenerate primers BC-dF and BC-dR. On the basis of this sequence information, two pairs of specific primers (BC-F1 and BC-R1, BC-F2 and BC-R2) were designed for 5' and 3' RACE, which generated a 1.7 kb fragment. The sequence of the fragment was determined as a fusion of the 5' and 3' ends of a putative BC cDNA. The coding region of BC gene was amplified using primers BC-F3 and BC-R3, which contains 1,743 bp encoding a deduced BC protein of 490 amino acid residues (GenBank accession No. GQ996717). Upstream of the translation start codon is a 107-bp 5' untranslated region and between the stop codon and poly (A) tail is a 3' untranslated region of 351 bp nucleotides. TGTAA, the sequence considered as a potential polyadenylation signal in green algae (Schmitt et al, 1992), is also found in the 3' untranslated region of the BC gene. The GC content of the BC coding region is 52.9%, which is much lower than GC content of BC genes from C. reinhardtii (67.8%) and H. pluvialis (63.7%). Protein sequence alignments showed that the BC of C. zofingiensis shared a high identity to that of 163 Chapter 9. Isolation and characterization of biotin carboxylase gene and stearoyl ACP desaturase gene from Chlorella zofingiensis other algae and higher plants, especially to the BC of H. pluvialis (74%) (Figure 9.1). The phylogenetic analysis indicates that the algal BC proteins are more related to those from plants (Figure 9.2). Figure 9.1 Amino acid sequence alignment of C. zofingiensis BC with that from other microalgae and plants. Amino acid residues which are either well or perfectly conserved in all sequences are indicated by (.) or (*) above the alignment, respectively. Primers are underlined with names. C. reinhardtii sequence (e_gwW.77.4.1) comes from ChlamyCenter (www.chlamy.org). Others from GeneBank: Brassica napus, AAK60339; Arabidopsis thaliana, CAA70282; Nicotiana tabacum, AAC41659 164 Chapter 9. Isolation and characterization of biotin carboxylase gene and stearoyl ACP desaturase gene from Chlorella zofingiensis Figure 9.2 Phylogenic tree of BC sequences from bacteria, algae and plants. A. arabaticum, Acetohalobium arabaticum (YP_003828313); E. guineensis, Elaeis guineensis (ABF74732); G. max, Glycine max (AAF80468); M. tuberculosis, Mycobacterium tuberculosis (NP_216238); R. communis, Ricinus communis (XP_002519811); S. coelicolor, Streptomyces coelicolor (NP_630967); S. linguale, Spirosoma linguale (ADB41216); others see Figure 9.1. Numbers associated with the branches are bootstrap values; Bootstrap was analyzed by using 1000 replicates. To characterize the corresponding gene of the BC cDNA, PCR using genomic DNA as the template was performed. A ca. 4.6 kb fragment was generated and sequenced. Analysis of the obtained nucleotide sequence revealed that the product was the corresponding gene of BC cDNA (GenBank accession No. GQ996718). The coding region of the BC gene is interrupted by eight introns of 346, 288, 377, 439, 339, 298, 358, 422 bp, respectively. Intron/exon splice sites of the BC gene are highly conservative, and all introns start with GT and end with AG. The primers for the isolation and characterization of C. zofingiensis SAD gene were shown in Table 9.1. The obtained SAD cDNA (GenBank accession No. GQ996719) contains 1,251 bp ORF that encodes a deduced SAD of 416 amino acid residues. The SAD protein shared a high homology with that from the near relative algae C. reinhardtii and H. pluvialis (Figure 9.3). The phylogenetic 165 Chapter 9. Isolation and characterization of biotin carboxylase gene and stearoyl ACP desaturase gene from Chlorella zofingiensis analysis indicates that the algal SAD proteins are more related to those from plants (Figure 9.4). The GC content of the SAD coding region is 53.2%, which is slightly higher than that of SAD from A. thaliana (47.9%) but lower than that from C. reinhardtii (64.0%) and H. pluvialis (60.4%). The coding region of SAD gene (GenBank accession No. GQ996720) is interrupted by six introns of 77, 224, 283, 264, 233, and 319 bp, respectively. Figure 9.3 Amino acid sequence alignment of C. zofingiensis SAD with its counterparts from C. reinhardtii (estExt_gwp_1W.C_130244), H. pluvialis (ABP57425), A. thaliana (AAK85232), B. napus (AAT65205) and Helianthus annuus (AAB65144). Amino acid residues which are either well or perfectly conserved in all sequences are indicated by (.) or (*) above the alignment, respectively. Primers are underlined with names. Conserved motifs are marked with boxes 166 Chapter 9. Isolation and characterization of biotin carboxylase gene and stearoyl ACP desaturase gene from Chlorella zofingiensis Figure 9.4 Phylogenic tree of BC sequences from bacteria, algae and plants. A. dehalogenans, Anaeromyxobacter dehalogenans (ZP_02322836); E. guineensis (AAB41041); G. max (ABM45912); M. tuberculosis (CAE55326); O. sativa, Oryza sativa (BAA07631); R. communis (XP_002526163); S. coelicolor (NP_630790); S. linguale (ADB40562); Solanum tuberosum, Solanum tuberosum (AAA33839); others see Figure 9.3. Numbers associated with the branches are bootstrap values; Bootstrap was analyzed by using 1000 replicates. 9.4.2 High light irradiation up-regulates the transcripts of BC and SAD and enhances the biosynthesis of TFA and oleic acid High light could induce the rapid accumulation of fatty acids in H. pluvialis (Zhekisheva et al., 2002, 2005; Chen, 2007). In the current study the effect of high light on the regulation of fatty acid biosynthesis in C. zofingiensis was assayed. The transcripts of BC and SAD genes of C. zofingiensis under high light condition were detected by RT-PCR analysis. The non-induced algal cells had basal BC transcripts (Figure 9.5A, lane 1). Upon exposure of the cells to high light illumination, a significant increase in the steady-state mRNA level was observed and the mRNA level reached its maximum at 24 h (Figure 9.5A, lanes 2-5). High light also up-regulated SAD transcripts, yet to much stronger extent as compared with BC transcripts (Figure 9.5A, lanes 2 and 3). A slight decrease of 167 Chapter 9. Isolation and characterization of biotin carboxylase gene and stearoyl ACP desaturase gene from Chlorella zofingiensis the steady-state SAD mRNA level was observed after 24 h upon onset of high light induction (Figure 9.5A, lanes 4 and 5). To correlate the transcript levels of BC and SAD genes and the biosynthesis of fatty acids, the accumulation of TFA and oleic acid was examined over the period of induction (Figure 9.5B). The Chlorella cells without light stress accumulated only a small amount of TFA (Figure 4B, 0 h). However, upon high light illumination, a great increase in the TFA content was observed (Figure 5B). Similarly, high light illumination strikingly stimulated the biosynthesis of oleic acid (Figure 9.5B). After 72 h of induction, the contents of TFA and oleic acid reached 19.3% and 3.1% of dry weight, 62.9% and 150.4% higher than that measured at 0 h, respectively. These results indicate that the up-regulation of BC leads to the rapid synthesis of TFA while the enhanced SAD expression increases the accumulation of oleic acid in C. zofingiensis cells. Figure 9.5 High light induced expression of BC and SAD genes (A) and accumulation of TFA and oleic acid (B) in C. zofingiensis cells. Dark column, TFA content; gray column, oleic acid content 168 Chapter 9. Isolation and characterization of biotin carboxylase gene and stearoyl ACP desaturase gene from Chlorella zofingiensis 9.4.3 Salt stress induces the up-regulation of SAD gene and the accumulation of oleic acid Salt was reported to be able to promote accumulation of TFA and affected the fatty acid composition in microalga (Rao et al., 2007). To examine the effect of salt on expression of BC and SAD genes and fatty acid biosynthesis, 100 mM NaCl was employed to treat C. zofingiensis cells. During the whole induction period, the mRNA level of BC stayed almost constant (Figure 9.6A, lanes 1-5). Correlated with the unchanged BC expression, no significant difference in TFA content was observed among the algal cell samples treated with salt for 0 to 72 h (Figure 9.6B). On the contrary, the SAD transcript was transiently up-regulated by salt and reached a maximum level at 24 h (Figure 9.6A). Accordingly, the oleic acid content increased upon induction of salt, and after 72 h of induction it reached 1.7% of dry weight, 39.0% higher than that measured at 0 h, yet much lower than that induced by high light (Figure 9.6B). 169 Chapter 9. Isolation and characterization of biotin carboxylase gene and stearoyl ACP desaturase gene from Chlorella zofingiensis Figure 9.6 Effect of salt stress on the expression of BC and SAD genes (A) and accumulation of TFA and oleic acid (B) in C. zofingiensis cells. Dark column, TFA content; gray column, oleic acid content 9.4.5 Glucose triggers the up-regulation of BC and SAD genes and induces the enhanced biosynthesis of TFA and oleic acid It was reported that glucose could stimulate the biosynthesis of fatty acids in C. zofingiensis (Chapter 6, 7 and 8). To survey the effect of glucose on fatty acid biosynthesis at transcriptional level, C. zofingiensis cells were induced by 50 mM glucose in the absence of light. The mRNA level of BC remained constant during the first 12 h of glucose induction and was then greatly up-regulated, reaching its maximum at 36 h (Figure 9.5A, lanes 1-4). Thereafter, a slight decrease of BC transcripts was observed (Figure 9.5A, lane 5). The mRNA level of SAD was also transiently up-regulated by glucose and began to decrease after 24 h of glucose induction (Figure 9.5A). Consistent with the up-regulation of BC and SAD genes, glucose enhanced the biosynthesis of TFA and oleic acid (Figure 9.5B). After 72 h of induction, the TFA content reached 29.3% of dry weight, which was 133.5% higher than that measured at 0 h and was 52.0% higher than that induced by high light. In contrast, after 72 h of glucose induction, more than 6-fold amounts of oleic acid accumulated, which was much higher than that induced by high light. Thus glucose may be superior to high light for inducing accumulation of TFA, especially of oleic acid in C. zofingiensis cells. 170 Chapter 9. Isolation and characterization of biotin carboxylase gene and stearoyl ACP desaturase gene from Chlorella zofingiensis Figure 9.5 Glucose induced expression of BC and SAD genes (A) and accumulation of TFA and oleic acid (B) in C. zofingiensis cells. Dark column, TFA content; gray column, oleic acid content 9.5 Discussion The biosynthesis of fatty acids has been thoroughly investigated at the molecular level in plants, whereas it is rarely touched in microalgae with a few exceptions (Roessler & Ohlrogge, 1993; Roessler et al., 1994; Sakamoto et al., 1994; Chen, 2007). This study reported the isolation and characterization from C. zofingiensis of two key genes (BC and SAD) involved in fatty acid biosynthesis. The deduced amino acid sequence of the BC cDNA from C. zofingiensis showed high identity to its counterparts from other algae and plants (Figure 9.1). BC is a subunit of ACCase, together with biotin carboxyl carrier protein and carboxyltransferase to constitute the multisubunit form of ACCase (Bao et al., 1997). The expression of BC is autoregulated to that of other subunits of ACCase 171 Chapter 9. Isolation and characterization of biotin carboxylase gene and stearoyl ACP desaturase gene from Chlorella zofingiensis and thus could represent the expression of ACCase (Ke et al., 2000; James & Cronan, 2004). SAD catalyzes the O2- and NAD(P)H-dependent insertion of a cis double bond between carbons 9 and 10 of 18:0-ACP to form 18:1-ACP (Sobrado et al., 2006). Similar to SADs from other algae and plants, the C. zofingiensis SAD contains two EXXH motifs that are considered conservative in the diiron-oxo protein class (Figure 9.2). In response to high light irradiation, BC and SAD genes were up-regulated in C. zofingiensis (Figure 9.3A); as a result, the treated cells accumulated a higher amount of TFA including oleic acid (Figure 9.3B). This might result from the overproduction of intracellular reactive oxygen species (ROS), because high light can stimulate a drastic increase ROS level in C. zofingiensis (Li et al., 2009). Further studies are needed to support this hypothesis. The enhanced de novo fatty acid synthesis consumes a large amount of oxygen, which in turn might serve as the ROS sequestration process and protect cells from damage by ROS (Chen, 2007). The increased intracellular ROS level was also observed in salt treated C. zofingiensis cells (Li et al., 2009). However, Unlike high light, salt stress only stimulated the up-regulation of SAD (Figure 9.4A); accordingly, the salt-treated cells accumulated a higher level of oleic acid whereas no significant difference in TFA content was observed as compared to non treated cells (Figure 9.4B). It was suggested that high light and salt stress might trigger the production of different ROS species that differentially stimulated the expression of BC and SAD genes. Glucose not only fuel cellular carbon and energy metabolism but also plays pivotal roles as signaling molecules, for example, to regulate genes involved in lipid biosynthesis (Rolland et al., 2006). This study showed that glucose could greatly up-regulate the expression of both BC and SAD genes and enhance the accumulation of TFA including oleic acid (Figure 9.5). It was suggested that glucose effects on the expression of genes might be mediated by glucose sensing, changes in electron flow of respiratory electron transport, or ROS (Moller, 2001; Moore et al., 2003; Ryu et al., 2004). Although glucose sensing and the 172 Chapter 9. Isolation and characterization of biotin carboxylase gene and stearoyl ACP desaturase gene from Chlorella zofingiensis mitochondrial alternative pathway were revealed to be involved in the regulation of carotenogenic genes of C. zofigiensis (Li et al., 2008b), the underlying mechanism of glucose effect on fatty acid biosynthesis genes remains open. The well studied pathway of fatty acid biosynthesis and the availability of genes involved in the fatty acid biosynthesis have greatly facilitated the genetic modification of plants for oil and fatty acid production, for example, to increase the total oil content (Roesler et al., 1997; Lardizabal et al., 2008), to change the composition of fatty acids (Flores et al., 2008; Graef et al., 2009), or to produce new fatty acids for nutritional improvement (Kinney et al., 2004; Cheng et al., 2010). Attempts to genetically manipulate microalgae through the induction of ACCase gene was carried out, with the hope that increasing the level of ACCase activity in the cells would lead to higher oil production (Sheehan et al., 1998). These early experiments did not, however, demonstrate increased oil production in the cells. Better understanding of regulatory processes of fatty acid biosynthesis and lipid metabolism and better genetic manipulation tools might be needed for the successful manipulation of microalga for enhanced oil production. The current study described for the first time the isolation and characterization of two important genes involved in the fatty acid biosynthesis, namely BC and SAD from C. zofingiensis. These results might help better understanding the fatty acid biosynthesis in C. zofingiensis at molecular level and benefit future genetic modification of this alga to enhance fatty acid production and/or alter fatty acid composition as a better biodiesel feedstock. 173 PART IV RESEARCH SUMMARY AND RECOMMENDATION FOR FUTURE WORK 174 Chapter 10. Research summary and recommendation for future work Chapter 10 Research summary and recommendation for future work 10.1 Introduction This thesis addressed the genetic engineering of Chlorella zofingiensis for promoted astaxanthin production and the assessment of heterotrophic C. zofingiensis as a biodiesel feedstock. Two approaches, namely, mutagenesis and manipulation of carotenoid biosynthesis, were employed to enhance the cellular astaxanthin content of C. zofingiensis. Lipid and fatty acid analyses were conducted to assess the biodiesel production potential of heterotrophic C. zofingiensis. 10.2 Research summary C. zofingiensis represents the fast growing green microalga with the ability of producing asatxanthin. To further increase the cellular astaxanthin content, mutagenesis of the alga was performed. Chemical mutagens MNNG (45 μg L-1) and EMS (0.36 M) were employed to treat the algal cells for generating mutations. Target mutants were isolated by screening the treated cells with 0.5 μM norflurazon. Of more than 200 mutants, 5 ones that exhibited deeper red intensity were selected for downstream characterization. No significant differences in cell growth and pigment profile were observed between the WT and mutants under normal growth condition without norflurazon. In contrast to WT cells that got bleached by 0.25 μM norflurazon, the mutants grew well and synthesized astaxanthin even when the culture medium contained up to 1 μM norflurazon. 175 Chapter 10. Research summary and recommendation for future work Furthermore, these mutants accumulated significant greater amounts of astaxanthin (up to 54% greater) than the WT when the cultures were induced with 30 g L-1 glucose in the dark for 5 days. Correlated with the higher levels of astaxanthin, the mutants showed higher transcript levels of BKT and CHYb genes that are directly involved in astaxanthin biosynthesis. Norflurazon specifically targets to phytoene desaturase (PDS) and inhibits its desaturation activity. The norflurazon resistance and enhanced astaxanthin accumulation of mutants might be attributed to certain mutations in PDS gene. To confirm this, the C. zofingiensis PDS gene should be first isolated and characterized. The open reading frame of this PDS gene, interrupted by six introns, encoded a polypeptide of 558 amino acid residues. The deduced protein sequence showed high homology with PDSs from other algae, cyanobacteria and higher plants. Expression of the PDS gene in E. coli demonstrated that the enzyme was able to convert phytoene to ζ-carotene efficiently. The PDS gene in C. zofingiensis was shown to be up-regulated by high light and glucose treatment. E17, one of the stable mutants, produced higher levels of total carotenoids (TC) and astaxanthin than the WT when induced by high light irradiation or glucose. A point mutation (C to T) was revealed to occur in the PDS gene of E17, leading to an amino acid change (L516F) in its coding region. The mutated PDS exhibited 31-fold resistance to norflurazon when compared to the WT one as determined by an in vitro assay. Surprisingly, the mutated PDS exhibited higher efficiency in converting phytoene to ζ-carotene. No difference in PDS transcripts was found between E17 and WT cells cultured either in normal or induced conditions. In contrast, higher transcript levels of BKT and CHYb were found in E17 cells. Therefore, it is concluded that the point mutation in the PDS gene makes E17 resistant against norflurazon and synthesize higher amounts of carotenoids including astaxanthin. Since the PDS gene from E17 encoded a enzyme resistant to norflurazon and having higher desaturation activity, it may be adopted as a dominant selectable marker for the transformation of C. zofingiensis. PDS-L516F gene was 176 Chapter 10. Research summary and recommendation for future work introduced into C. zofingiensis via the biolistic approach. Transformants expressing the mutated PDS gene showed strong resistance to the herbicide norflurazon. The transformant P6 could accumulate more TC and astaxanthin than the WT when cultured with glucose in the dark. The enhanced accumulation of TC and astaxanthin in the transformant was revealed to be related to the increase of PDS transcript. These results clearly show that the mutated PDS gene is a useful selectable marker and can be used to genetic engineer carotenoid biosynthesis for enhanced astaxanthin production in C. zofingiensis. C. zofingiensis also represents the oil-rich green alga that can grow well photoautotrophicaly, heterotrophically and mixotrophically. Profiles of algal lipids and fatty acids, which depend on species and culture conditions, are important data to evaluate the oils for biodiesel production. So, firstly, the lipid class and fatty acid composition of C. zofingiensis cultivated under photoautotrophic and heterotrophic conditions were documented and compared. Compared with photoautotrophic cells, a 900% increase in lipid yield was achieved in heterotrophic cells fed with 30 g L-1 of glucose. Furthermore heterotrophic cells accumulated predominantly neutral lipids (NL) that accounted for 79.5% of total lipids with 88.7% of NL being triacylglycerol (TAG); whereas photoautotrophic cells contained mainly the membrane lipids glycolipids (GL) and phospholipids (PL). Together with the much higher content of oleic acid (C18:1, 35.2% of total fatty acids), oils from heterotrophic C. zofingiensis appear to be more feasible for biodiesel production. Secondly, the lipid production and fatty acid profile of C. zofingiensis cultured in the dark with various carbon sources were investigated. Of the sugars surveyed, glucose was found to be the best one for the growth and lipid production. When cultivated with 50 g L-1 glucose, C. zofingiensis accumulated lipids up to 52% of the dry biomass, with TAG accounting for 72.1% of the total lipids. Fatty acid profiles revealed that glucose contributed to the highest yield of total fatty acids (TFA) and proportion of oleic acid (35.7% of TFA). In fed-batch cultivation based on glucose, the lipid yield and productivity of C. zofingiensis 177 Chapter 10. Research summary and recommendation for future work were further increased to 20.7 g L-1 and 1.38 g d-1 L-1 respectively, much higher than those achieved in batch culture. Moreover, the low cost cane molasses was surveyed and proved to be an ideal carbon source for boosting lipid production by C. zofingensis. These results suggest that C. zofingiensis has great potential for biodiesel production. In addition, the effect of several nutrients, namely, nitrate, phosphate and ferrous ion as well as the environmental conditions including temperature and the initial pH of culture medium on the growth and fatty acid profile of heterotrophic C. zofingiensis in batch culture were surveyed. The optimal nutritional and environmental conditions for TFA production by C. zofingiensis were 5 mM nitrate, 5 mM phosphate, 25-100 μM ferrous ion, cultivation temperature of 25 °C and the initial pH of 6.5. In order to elucidate the molecular regulation of fatty acid accumulation, the key genes involved in fatty acid biosynthesis, namely, biotin carboxylase (BC) and stearoyl ACP desaturase (SAD) genes, were isolated and characterized from C. zofingiensis. Protein sequence alignments showed that the deduced amino acid sequences of these two genes shared high identity to that of corresponding genes from other microalgae and plants. High light illumination and glucose induction were revealed able to up-regulate the expression of both BC and SAD genes, and therefore enhanced the accumulation of TFA including oleic acid. Unlike high light or glucose, salt stress, however, could only trigger the up-regulation of SAD gene; accordingly, the TFA content was slight affected while the biosynthesis of oleic acid was promoted. In this context, the fatty acid biosynthesis of C. zofingiensis might be regulated, at least partly, at the transcriptional level. 178 Chapter 10. Research summary and recommendation for future work 10.3 Recommendation for future work 10.3.1 Future work for astaxanthin production by C. zofingiensis It was reported in chapter 3 that the mutagenesis of C. zofingiensis coupled with norflurazon selection gave rise to the mutants with the ability of producing higher amounts of TC including astaxanthin. Following this strategy, the cells of mutant E17 can be further mutagenized but selected with other herbicides that target different carotenogenic enzymes. For example, diphenylamine, a specific inhibitor to carotenoid ketolase (Zhekisheva et al., 2005; Wang et al., 2008), can be adopted to select the mutants showing improved oxygenation activity of converting zeaxanthin to astaxanthin. Another feasible way to further increase the cellular astaxanthin content is directly engineering the astaxanthin biosynthetic pathway in C. zofingiensis. Under induced conditions for carotenoid biosynthesis, apart from astaxanthin, C. zofingiensis also accumulated substantial amounts of other secondary carotenoids such as adonixanthin and canthaxanthin (Rise et al., 1994; Bar et al., 1995; Del Campo et al., 2004; Wang et al., 2008). It might be possible that CHYb cannot accept canthaxanthin as a substrate to produce astaxanthin and BKT has a relatively low activity of hydroxylating adonixanthin to astaxanthin in C. zofingiensis. Therefore, the astaxanthin biosynthetic pathway in C. zofingiensis was proposed (Li et al., 2008b; Wang et al., 2008) and is shown in Figure 10.1. By introducing a carotenoid hydroxylase that can convert canthaxanthin to astaxanthin and a carotenoid ketolase that can catalyze the efficient ketolation of adonixanthin to astaxanthin, the secondary carotenoid flux in C. zofingiensis would be changed and directed to the only end product astaxanthin, resulting in further much enhanced astaxanthin production. The major drawback of using heterotrophic C. zofingiensis for astaxanthin production is the need of glucose to feed the cells because of the relatively high cost of glucose. It was reported in chapter 7 that cane molasses, the 179 Chapter 10. Research summary and recommendation for future work low cost by-product from sugar industry, could be well used as the carbon source for the growth of heterotrophic C. zofingiensis. Thus, it is reasonable to expect that cane molasses can be adopted to feed C. zofingiensis for economic production of astaxanthin. Phytoene PDS β-carotene BKT CHYb β-cryptoxanthin Echinenone CHYb BKT Zeaxanthin Canthaxanthin BKT Adonixanthin BKT Astaxanthin Figure 10.1 The proposed astaxanthin biosynthetic pathway in C. zofingiens. Enzymes are named according to the designation of their genes. PDS, phytoene desaturase; BKT, carotenoid ketolase; CHYb, carotenoid hydroxylase. 10.3.2 Future work for biodiesel production by C. zofingiensis C. zofingiensis can accumulate oils up to 52% of dry weight in heterotrophic conditions (chapter 7). The oils are composed almost exclusively of TAG, but they do contain small amounts of other lipidic compounds such as PL and GL. TAG consist of three fatty acid chains bound to a glycerol backbone. As 180 Chapter 10. Research summary and recommendation for future work such, theoretically, genetic engineering for cellular oil enhancement can be achieved through either increasing the synthesis of fatty acids or increasing their incorporation onto the glycerol backbone. In the former case, this involves attempts to enhance the partitioning of carbon toward fatty acid synthesis and to increase the pool sizes of substrates for fatty acid synthesis, through the overexpression of key metabolic enzymes, such as acetyl-CoA carboxylase (Roesler et al., 1997). As for the later, diacylglycerol acyltransferase, the enzyme catalyzes the last step of TAG biosynthesis - the incorporation of a fatty acyl-CoA onto diacylglycerol, is centred and manipulated (Lardizabal et al., 2008). While the oil enhancement in a feedstock severs a guide for better yield of biodiesel, the genetic modification of fatty acid composition points to the better quality of biodiesel. It has been suggested that the enhanced proportion of oleic acid gives biodiesel a compromise between oxidative stability and low-temperature properties and thus promotes biodiesel quality (Knothe, 2009). Oleic acid is converted to linoleic acid in a single desaturation step carried out by △12 fatty acid desaturase encoded by the FAD2 gene (Graef et al., 2009). Down-regulation of FAD2 through posttranscriptional gene-silencing methods results in much higher levels of oleic acid in soybean seeds (Buhr et al., 2002; Graef et al., 2009), which may provide a guide for enhanced proportion of oleic acid in C. zofingiensis. In this thesis, the potential assessment of C. zofingiensis as a biodiesel feedstock focused on the lipid and fatty acid analyses. Further explorations can be carried out, in terms of transesterification of C. zofingiensis derived oils for producing biodiesel and analysis of the biodiesel properties including density, viscosity, flash point, cloud point, cold-filter plugging point, heating value, oxidative stability, etc. Preliminary results suggested that the accumulation of lipids/fatty acids was accompanied by the accumulation of astaxanthin in heterotrophic C. zofingiensis. The underlying mechanism remains largely unknown and needs to be further investigated. This also stimulates the idea of simultaneous production of oils and astaxanthin by C. zofingiensis in the heterotrophic conditions, which, 181 Chapter 10. Research summary and recommendation for future work once implemented, will cut down the production cost and make this alga more commercially economical. 182 REFERENCES Aaronson S (1973) Effect of incubation temperature on the macromolecular and lipid content of the phytoflagellate Ochromonas danica. J Phycol 9: 111-113 Abdullah AZ, Salamatinia B, Mootabadi H, Bhatia S (2009) Current status and policies on biodiesel industry in Malaysia as the world's leading producer of palm oil. Energy Policy 37: 5440-5448 Ako H, Tamaru CS (1999) Are feeds for food fish practical for aquarium fish? Intl Aqua Feed 2: 30-36 Al-Babili S, Hugueney P, Schledz M, Welsch R, Frohnmeyer H, Laule O, Beyer P (2000) Identification of a novel gene coding for neoxanthin synthase from Solanum tuberosum. FEBS Lett 485: 168-172 Al-Babili S, VonLintig J, Haubruck H, Beyer P (1996) A novel, soluble form of phytoene desaturase from Narcissus pseudonarcissus chromoplasts is Hsp70-complexed and competent for flavinylation, membrane association and enzymatic activation. Plant J 9: 601-612 Albrecht M, Misawa N, Sandmann G (1999) Metabolic engineering of the terpenoid biosynthetic pathway of Escherichia coli for production of the carotenoids beta-carotene and zeaxanthin. Biotechnol Lett 21: 791-795 AlgaTechnologies (2004) Astaxanthin - a superb natural antioxidant. http://www.algatech.com/astax.htm Alonso DL, Belarbi EH, Fernandez-Sevilla JM, Rodriguez-Ruiz J, Grima EM (2000) Acyl lipid composition variation related to culture age and nitrogen concentration in continuous culture of the microalga Phaeodactylum tricornutum. Phytochem 54: 461-471 Al-Widyan MI, Al-Shyoukh AO (2002) Experimental evaluation of the transesterification of waste palm oil into biodiesel. Bioresour Technol 85: 253-256 An GH, Schuman DB, Johnson EA (1989) Isolation of Phaffia rhodozyma 183 mutants with increased astaxanthin content. Appl Environ Microb 55: 116-124 An G-H, Jang B-G, carotenoid-hyperproducing Cho M-H mutant (2001) 2A2N Cultivation of the of red the yeast Xanthophyllomyces dendrorhous (Phaffia rhodozyma) with molasses. J Biosci Bioeng 92: 121-125 An G-H, Kim C-H, Choi E-S, Rhee S-K (1996) Medium optimization for cultivation of carotenoid hyperproducing Phaffia rhodozyma mutant HT-5FO1C. J Ferment Bioeng 82: 515-518 Ando S, Tanaka Y (1996) Carotenoid forms in the exoskeketon of crayfish and kuruma prawn. Mem Fac Fish Kagoshima Univ 45: 5-12 Arias RS, Dayan FE, Michel A, Howell J, Scheffler BE (2006) Characterization of a higher plant herbicide-resistant phytoene desaturase and its use as a selectable marker. Plant Biotechnol J 4: 263-273 Armstrong GA (1997) Genetics of eubacterial carotenoid biosynthesis: A colorful tale. Annu Rev Microbiol 51: 629-659 Ataya F, Dube MA, Ternan M (2007) Acid-catalyzed transesterification of canola oil to biodiesel under single- and two-phase reaction conditions. Energ Fuel 21: 2450-2459 Ausich RL (1997) Commercial opportunities for carotenoid production by biotechnology. Pure Appl Chem 69: 2169-2173 Babu CM, Chakrabarti R, Surya Sambasivarao KR (2008) Enzymatic isolation of carotenoid-protein complex from shrimp head waste and its use as a source of carotenoids. LWT - Food Sci Technol 41: 227-235 Banerjee A, Chakraborty R (2009) Parametric sensitivity in transesterification of waste cooking oil for biodiesel production--A review. Resour Conserv Recycl 53: 490-497 Bao X, Shorrosh BS, Ohlrogge JB (1997) Isolation and characterization of an Arabidopsis biotin carboxylase gene and its promoter. Plant Mol Biol 35: 539-550 184 Bar E, Rise M, Vishkautsan M, Arad S (1995) Pigments and structural changes in Chlorella zofingiensis upon light and nitrogen stress. J Plant Physiol 146: 527-534 Barros MP, Pinto E, Colepicolo P, Pedersen M (2001) Astaxanthin and peridinin inhibit oxidative damage in Fe2+-loaded liposomes: Scavenging oxyradicals or changing membrane permeability? Biochem Bioph Res Co 288: 225-232 Bennedsen M, Wang X, Willen R, Wadstron T, Andersen LP (2000) Treatment of H. pylori infected mice with antioxidant astaxanthin reduces gastric inflammation, bacterial load and modulates cytokine release by splenocytes. Immunol Lett 70: 185-189 Bjerkeng B, Peisker M, von Schwartzenberg K, Ytrestoyl T, Asgard T (2007) Digestibility and muscle retention of astaxanthin in Atlantic salmon, Salmo salar, fed diets with the red yeast Phaffia rhodozyma in comparison with synthetic formulated astaxanthin. Aquaculture 269: 476-489 Bon JA, Leathers TD, Jayaswal RK (1997) Isolation of astaxanthin-overproducing mutants of Phaffia rhodozyma. Biotechnol Lett 19: 109-112 Bonk M, Hoffmann B, VonLintig J, Schledz M, AlBabili S, Hobeika E, Kleinig H, Beyer P (1997) Chloroplast import of four carotenoid biosynthetic enzymes in vitro reveals differential fates prior to membrane binding and oligomeric assembly. Eur J Biochem 247: 942-950 Boussiba S (2000) Carotenogenesis in the green alga Haematococcus pluvialis: Cellular physiology and stress response. Physiol Plant 108: 111-117 Boussiba S, Fan L, Vonshak A (1992) Enhancement and determination of astaxanthin accumulation in green alga Haematococcus pluvialis. In: Packer. L (ed) Methods in Enzymology, Carotenoids Part A 213. Academic Press, London, pp 371-386 Boussiba S, Vonshak A, Cohen Z, Avissar Y, Richmond A (1987) Lipid and biomass production by the halotolerant microalga Nannochloropsis salina. Biomass 12: 37-47 Bouvier F, d'Harlingue A, Hugueney P, Marin E, Marion-Poll A, Camara B (1996) 185 Xanthophyll biosynthesis. J Biol Chem 271: 28861-28867 Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248-254 Breitenbach J, Misawa N, Kajiwara S, Sandmann G (1996) Expression in Escherichia coli and properties of the carotene ketolase from Haematococcus pluvialis. FEMS Microbiol Lett 140: 241-246 Breitenbach J, Zhu CF, Sandmann G (2001) Bleaching herbicide norflurazon inhibits phytoene desaturase by competition with the cofactors. J Agr Food Chem 49: 5270-5272 Brennan L, Owende P (2010) Biofuels from microalgae--A review of technologies for production, processing, and extractions of biofuels and co-products. Renew Sustain Energy Rev 14: 557-577 Britton G (1995) Structure and properties of carotenoids in relation to function. FASEB J 9: 1551-1558 Britton G, Liaaen-Jensen S, Pfander H (2004) Carotenoids handbook. Birkhauser Verlag Britton G, Weesie RJ, Askin D, Warburton JD, GallardoGuerrero L, Jansen FJ, deGroot HJM, Lugtenburg J, Cornard JP, Merlin JC (1997) Carotenoid blues: Structural studies on carotenoproteins. Pure Appl Chem 69: 2075-2084 Brown MR, Dunstan GA, Norwood SJ, Miller KA (1996) Effects of harvest stage and light on the biochemical composition of the diatom Thalassiosira pseudonana. J Phycol 32: 64-73 Buhr T, Sato S, Ebrahim F, Xing A, Zhou Y, Mathiesen M, Schweiger B, Kinney A, Staswick P (2002) Ribozyme termination of RNA transcripts down-regulate seed fatty acid genes in transgenic soybean. Plant J 30: 155-163 Canakci M (2007) The potential of restaurant waste lipids as biodiesel feedstocks. Bioresour Technol 98: 183-190 Carvalho AP, Meireles LA, Malcata FX (2006) Microalgal reactors: A review of 186 enclosed system designs and performances. Biotechnol Progr 22: 1490-1506 Cerutti H, Johnson AM, Gillham NW, Boynton JE (1997) Epigenetic silencing of a foreign gene in nuclear transformants of Chlamydomonas. Plant Cell 9: 925-945 Chamovitz D, Pecker I, Hirschberg J (1991) The molecular-basis of resistance to the herbicide norflurazon. Plant Mol Biol 16: 967-974 Chamovitz D, Sandmann G, Hirschberg J (1993) Molecular and biochemical-characterization of herbicide-resistant mutants of cyanobacteria reveals that phytoene desaturation is a rate-limiting step in carotenoid biosynthesis. J Biol Chem 268: 17348-17353 Checkbiotech (2009) Massive increase in global biofuel production. http://bioenergy.checkbiotech.org/news/massive_increase_global_biofuel_pr oduction Chen F (1996) High cell density culture of microalgae in heterotrophic growth. Trends Biotechnol 14: 421-426 Chen G (2007) Lipid and fatty acid composition and the biosynthesis in relation to carotenoid accumulation in the microalgae Nitzschia laevis (Bacillariophyceae) and Haematococcus pluvialis (Chlorophyceae). The University of Hong Kong, Hong Kong Chen G-Q, Jiang Y, Chen F (2007) Fatty acid and lipid class composition of the eicosapentaenoic acid-producing microalga, Nitzschia laevis. Food Chem 104: 1580-1585 Chen G-Q, Jiang Y, Chen F (2008) Salt-induced alterations in lipid composition of diatom Nitzshia laevis (Bacillariophyceae). J Phycol 44: 1309-1314 Chen Y, Li D, Lu W, Xing J, Hui B, Han Y (2003) Screening and characterization of astaxanthin-hyperproducing mutants of Haematococcus pluvialis. Biotechnol Lett 25: 527-529 Cheng B, Wu G, Vrinten P, Falk K, Bauer J, Qiu X (2010) Towards the production of high levels of eicosapentaenoic acid in transgenic plants: the effects of different host species, genes and promoters. Transgenic Res 19: 187 221-229 Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25: 294-306 Chisti Y (2008) Biodiesel from microalgae beats bioethanol. Trends Biotechnol 26: 126-131 Choubert G, Baccaunaud M (2010) Effect of moist or dry heat cooking procedures on carotenoid retention and colour of fillets of rainbow trout (Oncorhynchus mykiss) fed astaxanthin or canthaxanthin. Food Chem 119: 265-269 Christie WW (2003) Lipid analysis: Isolation, separatoin, identification, and structural analysis of lipids. The Oily Press, Bridgwater, England Cleber Bertoldi F, Sant'anna E, Braga MVDC, Luiz Barcelos Ollveira J (2006) Lipids, fatty acids composition and carotenoids of Chlorella vulgaris cultivated in hydroponic wastewater. Grasas Y Aceites 57: 270-274 Cruz JM, Parajo JC (1998) Improved astaxanthin production by Xanthophyllomyces dendrorhous growing on enzymatic wood hydrolysates containing glucose and cellobiose. Food Chem 63: 479-484 Cunningham FX, Gantt E (1998) Genes and enzymes of carotenoid biosynthesis in plants. Annu Rev Plant Phys 49: 557-583 Cunningham FX, Gantt E (2001) One ring or two? Determination of ring number in carotenoids by lycopene ε-cyclases. Proc Natl Acad Sci USA 98: 2905-2910 Cunningham Jr FX, Sun Z, Chamovitz D, Hirschberg J, Gantt E (1994) Molecular structure and enzymatic function of lycopene cyclase from the cyanobacterium Synechococcus sp strain PCC7942. Plant Cell 6: 1107-1121 Curek GD, Cort A, Yucel G, Demir N, Ozturk S, Elpek GO, Savas B, Aslan M (2010) Effect of astaxanthin on hepatocellular injury following ischemia/reperfusion. Toxicology 267: 147-153 da Cunha ME, Krause LC, Moraes MSA, Faccini CS, Jacques RA, Almeida SR, Rodrigues MRA, Caramao EB (2009) Beef tallow biodiesel produced in a pilot scale. Fuel Process Technol 90: 570-575 188 Dahlqvist A, Stahl U, Lenman M, Banas A, Lee M, Sandager L, Ronne H, Stymne H (2000) Phospholipid : diacylglycerol acyltransferase: An enzyme that catalyzes the acyl-CoA-independent formation of triacylglycerol in yeast and plants. Proc Natl Acad Sci USA 97: 6487-6492 Damiani MC, Popovich CA, Constenla D, Leonardi PI (2010) Lipid analysis in Haematococcus pluvialis to assess its potential use as a biodiesel feedstock. Bioresour Technol 101: 3801-3807 de Castro Araujo S, Garcia VMT (2005) Growth and biochemical composition of the diatom Chaetoceros cf. wighamii brightwell under different temperature, salinity and carbon dioxide levels. I. Protein, carbohydrates and lipids. Aquaculture 246: 405-412 de Oliveira D, Di Luccio M, Faccio C, Dalla Rosa C, Bender J, Lipke N, Amroginski C, Dariva C, de Oliveira J (2005) Optimization of alkaline transesterification of soybean oil and castor oil for biodiesel production. Appl Biochem Biotechnol 122: 553-560 Del Campo JA, Rodriguez H, Moreno J, Vargas MA, Rivas J, Guerrero MG (2004) Accumulation of astaxanthin and lutein in Chlorella zofingiensis (Chlorophyta). Appl Microbiol Biotechnol 64: 848-854 Demirbas A (2002) Biodiesel from vegetable oils via transesterification in supercritical methanol. Energ Convers Manage 43: 2349-2356 Demirbas A (2005) Biodiesel production from vegetable oils via catalytic and non-catalytic supercritical methanol transesterification methods. Prog Energy Combust Sci 31: 466-487 Demirbas A (2008) Biodiesel-a realistic fuel alternative for diesel engines. Springer - Verlag, London Demirbas A (2009) Progress and recent trends in biodiesel fuels. Energ Convers Manage 50: 14-34 Demming-Adams B, Adams WW (1993) The xanthophyll cycle, protein turnover, and the high light tolerance of sun-acclimated leaves. Plant Physiol 103: 1413-1420 189 Dias JM, Alvim-Ferraz MCM, Almeida MF (2009) Production of biodiesel from acid waste lard. Bioresour Technol 100: 6355-6361 Diaz-Felix W, Riley MR, Zimmt W, Kazz M (2009) Pretreatment of yellow grease for efficient production of fatty acid methyl esters. Biom Bioenerg 33: 558-563 Dogbo O, Laferriere A, Dharlingue A, Camara B (1988) Carotenoid biosynthesis isolation and characterization of a bifunctional enzyme catalyzing the synthesis of phytoene. Proc Natl Acad Sci USA 85: 7054-7058 Elenkov I, Stefanov K, Dimitrova-Konaklieva S, Popov S (1996) Effect of salinity on lipid composition of Cladophora vagabunda. Phytochem 42: 39-44 Elton-Bott RR (1979) A re-investigation of phenol as a spectrophotometric reagent for the determination of nitrate-nitrogen. Analytica Chimica Acta 108: 285-291 Elwinger K, Lignell A, Wilhelmson M (1997) Astaxanthin rich algal meal (Haematococcus pluvialis) as carotenoid source in feed for laying hens. Proceedings of the VII European Symposium on the Quality of Eggs and Egg Products, Poznan, Poland, pp 52-59 Fabregas J, Maseda A, Domínguez A, Otero A (2004) The cell composition of Nannochloropsis sp. changes under different irradiances in semicontinuous culture. World J Microbiol Biotechnol 20: 31-35 Fabregas J, Otero A, Maseda A, Dominguez A (2001) Two-stage cultures for the production of astaxanthin from Haematococcus pluvialis. J Biotechnol 89: 65-71 Fan K-W, Jiang Y, Faan Y-W, Chen F (2007) Lipid characterization of Mangrove thraustochytrid - Schizochytrium mangrovei. J Agr Food Chem 55: 2906-2910 Fernandez-Gonzalez B, Sandmann G, Vioque A (1997) A new type of asymmetrically acting β-carotene ketolase is required for the synthesis of echinenone in the cyanobacterium Synechocystis sp. PCC 6803. J Biol Chem 272: 9728-9733 190 Flores T, Karpova O, Su XJ, Zeng PY, Bilyeu K, Sleper DA, Nguyen HT, Zhang ZJ (2008) Silencing of GmFAD3 gene by siRNA leads to low alpha-linolenic acids (18:3) of fad3-mutant phenotype in soybean Glycine max (Merr.). Transgenic Res 17: 839-850 Floreto EAT, Teshima S, Ishikawa M (1996) Effects of nitrogen and phosphorus on the growth and fatty acid composition of Ulva pertusa Kjellman (Chlorophyta). Botanica Marina 39: 69-74 Fraser PD, Bramley PM (2004) The biosynthesis and nutritional uses of carotenoids. Prog Lipid Res 43: 228-265 Fraser PD, Miura Y, Misawa N (1997) In vitro characterization of astaxanthin biosynthetic enzymes. J Biol Chem 272: 6128-6135 Fraser PD, Romer S, Shipton CA, Mills PB, Kiano JW, Misawa N, Drake RG, Schuch W, Bramley PM (2002) Evaluation of transgenic tomato plants expressing an additional phytoene synthase in a fruit-specific manner. Proc Natl Acad Sci USA 99: 1092-1097 Fraser PD, Schuch W, Bramley PM (2000) Phytoene synthase from tomato (Lycopersicon esculentum) chloroplasts - partial purification and biochemical properties. Planta 211: 361-369 Fraser PD, Shimada H, Misawa N (1998) Enzymic confirmation of reactions involved in routes to astaxanthin formation, elucidated using a direct substrate in vitro assay. Eur J Biochem 252: 229-236 Fredriksson S, Elwinger K, Pickova J (2006) Fatty acid and carotenoid composition of egg yolk as an effect of microalgae addition to feed formula for laying hens. Food Chem 99: 530-537 Fukuda H, Kondo A, Noda H (2001) Biodiesel fuel production by transesterification of oils. J Biosci Bioeng 92: 405-416 Furukawa S, Uehara Y, Yamasaki H (2010) Variables affecting the reactivity of acid-catalyzed transesterification of vegetable oil with methanol. Bioresour Technol 101: 3325-3332 Gao C, Zhai Y, Ding Y, Wu Q (2010) Application of sweet sorghum for biodiesel 191 production by heterotrophic microalga Chlorella protothecoides. Appl Energ 87: 756-761 Gerjets T, Sandmann G (2006) Ketocarotenoid formation in transgenic potato. J Exp Bot 57: 3639-3645 Gerjets T, Sandmann M, Zhu C, Sandmann G (2007) Metabolic engineering of ketocarotenoid biosynthesis in leaves and flowers of tobacco species. Biotechnol J 2: 1263-1269 Gerpen JV (2005) Biodiesel processing and production. Fuel Process Technol 86: 1097-1107 Gong X, Chen F (1997) Optimization of culture medium for growth of Haematococcus pluvialis. J Appl Phycol 9: 437-444 Goto S, Kogure K, Abe K, Kimata Y, Kitahama K, Yamashita E, Terada H (2001) Efficient radical trapping at the surface and inside the phospholipid membrane is responsible for highly potent antiperoxidative activity of the carotenoid astaxanthin. Biochim Biophys Acta 1512: 251-258 Gouda M, Omar S, Aouad L (2008) Single cell oil production by Gordonia sp. DG using agro-industrial wastes. World J Microbiol Biotechnol 24: 1703-1711 Gouveia L, Batista AP, Sousa I, Raymundo A, Bandarra NM (2009) Microalgae in novel food products. In: Hagen KN (ed) Algae: Nutrition, pollution control and energy sources. Nova Science Publishers, New yourk, pp 265-300 Gradelet S, Le Bon AM, Berges R, Suschetet M, Astorg P (1998) Dietary carotenoids inhibit aflatoxin B-1-induced liver preneoplastic foci and DNA damage in the rat: Role of the modulation of aflatoxin B-1 metabolism. Carcinogenesis 19: 403-411 Graef G, LaVallee BJ, Tenopir P, Tat M, Schweiger B, Kinney AJ, Gerpen JHV, Clemente TE (2009) A high-oleic-acid and low-palmitic-acid soybean: agronomic performance and evaluation as a feedstock for biodiesel. Plant Biotechnol J 7: 411-421 192 Graham LE, Wilcox LW, Graham J (2009) Algae. Benjamin Cummings, San Francisco, CA Greenwell HC, Laurens LML, Shields RJ, Lovitt RW, Flynn KJ (2009) Placing microalgae on the biofuels priority list: a review of the technological challenges. J R Soc Interface doi:10.1098/rsif.2009.0322 Grima EM, Fernandez FGA, Camacho FG, Chisti Y (1999) Photobioreactors: light regime, mass transfer, and scaleup. J Biotechnol 70: 231-247 Grunewald K, Eckert M, Hirschberg J, Hagen C (2000) Phytoene desaturase is localized exclusively in the chloroplast and up-regulated at the mRNA level during accumulation of secondary carotenoids in Haematococcus pluvialis (Volvocales, Chlorophyceae). Plant Physiol 122: 1261-1268 Grunewald K, Hagen C (2001) beta-carotene is the intermediate exported from the chloroplast during accumulation of secondary carotenoids in Haematococcus pluvialis. J Appl Phycol 13: 89-93 Grunewald K, Hirschberg J, Hagen C (2001) Ketocarotenoid biosynthesis outside of plastids in the unicellular green alga Haematococcus pluvialis. J Biol Chem 276: 6023-6029 Guan G, Kusakabe K, Sakurai N, Moriyama K (2009) Transesterification of vegetable oil to biodiesel fuel using acid catalysts in the presence of dimethyl ether. Fuel 88: 81-86 Guerin M, Huntley ME, Olaizola M (2003) Haematococcus astaxanthin: applications for human health and nutrition. Trends Biotechnol 21: 210-216 Handayani AD, Sutrisno, Indraswati N, Ismadji S (2008) Extraction of astaxanthin from giant tiger (Panaeus monodon) shrimp waste using palm oil: Studies of extraction kinetics and thermodynamic. Bioresour Technol 99: 4414-4419 Harker M, Hirschberg J (1997) Biosynthesis of ketocarotenoids in transgenic cyanobacteria expressing the algal gene for beta-C-4-oxygenase, crtO. FEBS Lett 404: 129-134 Harker M, Tsavalos AJ, Young AJ (1996) Autotrophic growth and carotenoid 193 production of Haematococcus pluvialis in a 30 liter air-lift photobioreactor. J Ferment Bioeng 82: 113-118 Harris RV, Harris P, James AT (1965) The fatty acid metabolism of Chlorella vulgaris. Biochim Biophys Acta 106: 465-473 Hasunuma T, Miyazawa SI, Yoshimura S, Shinzaki Y, Tomizawa KI, Shindo K, Choi SK, Misawa N, Miyake C (2008) Biosynthesis of astaxanthin in tobacco leaves by transplastomic engineering. Plant J 55: 857 - 868 Hata N, Ogbonna JC, Hasegawa Y, Taroda H, Tanaka H (2001) Production of astaxanthin by Haematococcus pluvialis in a sequential heterotrophic-photoautotrophic culture. J Appl Phycol 13: 395-402 Hawash S, Kamal N, Zaher F, Kenawi O, Diwani GE (2009) Biodiesel fuel from Jatropha oil via non-catalytic supercritical methanol transesterification. Fuel 88: 579-582 Henderson RJ, Mackinlay EE (1989) Effect of temperature on lipid composition of the marine cryptomonad Chroomonas salina. Phytochem 28: 2943-2948 Heu M-S, Kim J-S, Shahidi F (2003) Components and nutritional quality of shrimp processing by-products. Food Chem 82: 235-242 Higuera-Ciapara I, eacute, lix-Valenzuela L, Goycoolea FM (2006) Astaxanthin: A Review of its Chemistry and Applications. Crit Rev Food Sci Nutr 46: 185-196 Hill J, Nelson E, Tilman D, Polasky S, Tiffany D (2006) Environmental, economic, and energetic costs and benefits of biodiesel and ethanol biofuels. Proc Natl Acad Sci USA 103: 11206-11210 Hirschberg J (1998) Molecular biology of carotenoid biosynthesis. In: Britton G, Liaaen-Jensen S, Pfander H (eds) Carotenoids. Birkhauser Verlag, Basel Hirschberg J, Ohad N, Pecker I, Rahat A (1987) Isolation and characterization of herbicide resistant mutants in the cyanobacterium Synechococcus R2. Z Naturforsch C 42: 758-761 Holtin K, Kuehnle M, Rehbein J, Schuler P, Nicholson G, Albert K (2009) Determination of astaxanthin and astaxanthin esters in the microalgae 194 Haematococcus pluvialis by LC-(APCI)MS and characterization of predominant carotenoid isomers by NMR spectroscopy. Anal Bioanal Chem 395: 1613-1622 Hsieh C-H, Wu W-T (2009) Cultivation of microalgae for oil production with a cultivation strategy of urea limitation. Bioresour Technol 100: 3921-3926 Hu Q (2004) Environmental effects on cell composition. In: Richmond A (ed) Handbook of microalgal culture. Blackwell, Oxford, pp 83-93 Hu Q, Sommerfeld M, Jarvis E, Ghirardi M, Posewitz M, Seibert M, Darzins A (2008b) Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J 54: 621-639 Hu ZY, Li YT, Sommerfeld M, Chen F, Hu Q (2008a) Enhanced protection against oxidative stress in an astaxanthin-overproduction Haematococcus mutant (Chlorophyceae). Eur J Phycol 43: 365-376 Huang G, Chen F, Wei D, Zhang X, Chen G (2010) Biodiesel production by microalgal biotechnology. Appl Energ 87: 38-46 Huang JC, Chen F (2006) Simultaneous amplification of 5' and 3' cDNA ends based on template-switching effect and inverse PCR. Biotechniques 40: 187-9 Huang JC, Liu J, Li YT, Chen F (2008) Isolation and characterization of the phytoene desaturase gene as a potential selective marker for genetic engineering of the astaxanthin-producing green alga Chlorella zofingiensis (Chlorophyta). J Phycol 44: 684-690 Huang JC, Wang Y, Sandmann G, Chen F (2006) Isolation and characterization of a carotenoid oxygenase gene from Chlorella zofingiensis (Chlorophyta). Appl Microbiol Biotechnol 71: 473-479 Hussein G, Nakamura M, Zhao Q, Iguchi T, Goto H, Sankawa U, Watanabe H (2005) Antihypertensive and neuroprotective effects of astaxanthin in experimental animals. Biol Pharm Bull 28: 47-52 Imamoglu E, Dalay MC, Sukan FV (2009) Influences of different stress media and high light intensities on accumulation of astaxanthin in the green alga 195 Haematococcus pluvialis. New Biotechnol 26: 199-204 Inoue K, Furbee KJ, Uratsu S, Kato M, Dandekar AM, Ikoma Y (2006) Catalytic activities and chloroplast import of carotenogenic enzymes from citrus. Physiol Plant 127: 561-570 Ip PF (2005) Elicitation of astaxanthin biosynthesis in dark-heterotrophic cultures of Chlorella zofingiensis. Botany. The University of Hong Kong, Hong Kong Ip PF, Chen F (2005) Production of astaxanthin by the green microalga Chlorella zofingiensis in the dark. Process Biochem 40: 733-738 Ip PF, Wong KH, Chen F (2004) Enhanced production of astaxanthin by the green microalga Chlorella zofingiensis in mixotrophic culture. Process Biochem 39: 1761-1766 Ishikawa E, Sansawa H, Abe H (2004) Isolation and characterization of a Chlorella mutant producing high amounts of chlorophyll and carotenoids. J Appl Phycol 16: 385-393 Jain S, Sharma MP (2010) Prospects of biodiesel from Jatropha in India: A review. Renew Sustain Energy Rev 14: 763-771 James ES, Cronan JE (2004) Expression of two Escherichia coli acetyl-CoA carboxylase subunits is autoregulated. J Biol Chem 279: 2520-2527 Jang H-D, Lin Y-Y, Yang S-S (2005) Effect of culture media and conditions on polyunsaturated fatty acids production by Mortierella alpina. Bioresour Technol 96: 1633-1644 Jayaraj J, Devlin R, Punja Z (2008) Metabolic engineering of novel ketocarotenoid production in carrot plants. Transgenic Res 17: 489-501 Jiang L, Wang J, Liang S, Wang X, Cen P, Xu Z (2009) Butyric acid fermentation in a fibrous bed bioreactor with immobilized Clostridium tyrobutyricum from cane molasses. Bioresour Technol 100: 3403-3409 Jin E, Polle JEW, Lee HK, Hyun SM, Chang M (2003) Xanthophylls in microalgae: From biosynthesis to biotechnological mass production and application. J Microbiol Biotechnol 13: 165-174 Johnson EA, An GH (1991) Astaxanthin from microbial sources. Crit Rev 196 Biotechnol 11: 297-326 Johnson EA, Schroeder WA (1995) Microbial carotenoids. Adv Biochem Eng Biotechnol 53: 119-178 Johnson MB, Wen Z (2009) Production of biodiesel fuel from the microalga Schizochytrium limacinum by direct transesterification of algal biomass. Energ Fuel 23: 5179-5183 Jyonouchi H, Sun S, Tomita Y, Gross MD (1995) Astaxanthin, a carotenoid without vitamin A activity, augments antibody responses in cultures Including T-helper cell clones and suboptimal doses of antigen. J Nutr 125: 2483-2492 Jyonouchi H, Zhang L, Gross M, Tomita Y (1994) Immunomodulating actions of carotenoids: enhancement of in vivo and in vitro antibody production to T-dependent antigens. Nutr Cancer 21: 47-58 Jyonouchi H, Zhang L, Tomita Y (1993) Studies of immunomodulating actions of carotenoids. II. Astaxanthin enhances in vitro antibody production to T-dependent antigens without facilitating polyclonal B-cell activation. Nutr Cancer 19: 269-280 Kajiwara S, Kakizono T, Saito T, Kondo K, Ohtani T, Nishio N, Nagai S, Misawa N (1995) Isolation and functional identification of a novel cDNA for astaxanthin biosynthesis from Haematococcus pluvialis, and astaxanthin synthesis in Escherichia coli. Plant Mol Biol 29: 343-352 Kalogiannis S, Iakovidou G, Liakopoulou-Kyriakides M, Kyriakidis DA, Skaracis GN (2003) Optimization of xanthan gum production by Xanthomonas campestris grown in molasses. Process Biochem 39: 249-256 Kalscheuer R, Stolting T, Steinbuchel A (2006) Microdiesel: Escherichia coli engineered for fuel production. Microbiology 152: 2529-2536 Kawachi M, Inouye I, Honda D, O'Kelly CJ, Bailey JC, Bidigare RR, Andersen RA (2002) The Pinguiophyceae classis nova, a new class of photosynthetic stramenopiles whose members produce large amounts of omega-3 fatty acids. Phycolog Res 50: 31-47 197 Ke J, Wen T-N, Nikolau BJ, Wurtele ES (2000) Coordinate regulation of the nuclear and plastidic genes coding for the subunits of the heteromeric acetyl-coenzyme A carboxylase. Plant Physiol 122: 1057-1072 Khozin-Goldberg I, Bigogno C, Shrestha P, Cohen Z (2002) Nitrogen starvation induces the accumulation of arachidonic acid in the freshwater green alga Parietochloris incisa (trebuxiophyceae). J Phycol 38: 991-994 Kinney AJ, Cahoon EB, Damude HG, Hitz WD, Kolar CW, Liu ZB (2004) Production of very long chain polyunsaturated fatty acids in oilseed plants. World Patent Application No. WO 2004/071467 Knothe G (2005) Introduction: what is biodiesel. In: Knothe G, Gerpen JV, Krahl J (eds) The biodiesel handbook. AOCS Press, Champaign, pp 1-3 Knothe G (2005b) Dependence of biodiesel fuel properties on the structure of fatty acid alkyl esters. Fuel Process Technol 86: 1059-1070 Knothe G (2008) "Designer" biodiesel: Optimizing fatty ester composition to improve fuel properties. Energ Fuel 22: 1358-1364 Knothe G (2009) Improving biodiesel fuel properties by modifying fatty ester composition. Energ Environ Sci 2: 759-766 Koskimies-Soininen K, Nyberg H (1987) Effects of temperature and light on the lipids of Sphagnum magellanicum. Phytochem 26: 2213-2221 Kotzamanidis C, Roukas T, Skaracis G (2002) Optimization of lactic acid production from beet molasses by Lactobacillus delbrueckii NCIMB 8130. World J Microbiol Biotechnol 18: 441-448 Kovar JL, Zhang J, Funke RP, Weeks DP (2002) Molecular analysis of the acetolactate synthase gene of Chlamydomonas reinhardtii and development of a genetically engineered gene as a dominant selectable marker for genetic transformation. Plant J 29: 109-117 Kuhl A, Lorenzen H (1964) Handling and culturing of Chlorella. In: Prescott DM (ed) Methods in cell physiology. Academic Press, New York and London, pp 152-187 Kuntz M, Romer S, Suire C, Hugueney P, Weil JH, Schantz R, Camara B (1992) 198 Identification of a cDNA for the plastid-located geranylgeranyl pyrophosphate synthase from Capsicum annuum: correlative increase in enzyme activity and transcript level during fruit ripening. Plant J 2: 25-34 Kupcinskas L, Lafolie P, Lignell A, Kiudelis G, Jonaitis L, Adamonis K, Andersen LP, Wadstron T (2008) Efficacy of the natural antioxidant astaxanthin in the treatment of functional dyspepsia in patients with or without Helicobacter pylori infection: A prospective, randomized, double blind, and placebo-controlled study. Phytomedicine 15: 391-399 Kurashige M, Okimasu E, Inoue M, Utsumi K (1990) Inhibition of oxidative injury of biological-membranes by astaxanthin. Physiol Chem Phys Med NMR 22: 27-38 Laird LM, John HS, Karl KT, Steve AT (2001) Salmonid farming. In: Steele JH, Turekian KK, Thorpe SA (eds) Encyclopedia of ocean sciences. Academic Press, Oxford, pp 2482-2487 Lang X, Dalai AK, Bakhshi NN, Reaney MJ, Hertz PB (2001) Preparation and characterization of bio-diesels from various bio-oils. Bioresour Technol 80: 53-62 Lange BM, Ghassemian M (2003) Genome organization in Arabidopsis thaliana: a survey for genes involved in isoprenoid and chlorophyll metabolism. Plant Mol Biol 51: 925-948 Lardizabal K, Effertz R, Levering C, Mai J, Pedroso MC, Jury T, Aasen E, Gruys K, Bennett K (2008) Expression of Umbelopsis ramanniana DGAT2A in seed increases oil in soybean. Plant Physiol. 148: 89-96 Lawlor SM, O'Brien NM (1995) Astaxanthin: Antioxidant effects in chicken embryo fibroblasts. Nutr Res 15: 1695-1704 Lee JH, Song MW, Park KM (2008) Fermentation kinetic studies for production of carotenoids by Xanthophyllomyces dendrorhous. J Biotechnol 136: S732-S732 Lee RE (2008) Phycology. Cambridge University Press, Cambridge Leung DYC, Guo Y (2006) Transesterification of neat and used frying oil: 199 Optimization for biodiesel production. Fuel Process Technol 87: 883-890 Lewis MJ, Ragot N, Berlant MC, Miranda M (1990) Selection of astaxanthin-overproducing mutants of Phaffia rhodozyma with β-Ionone. Appl Environ Microbiol 56: 2944-2945 Li M, Gong R, Rao X, Liu Z, Wang X (2005) Effects of nitrate concentration on growth and fatty acid composition of the marine microalga Pavlova viridis (Prymnesiophyceae). Ann Microbiol 55: 51-55 Li Q, Du W, Liu DH (2008c) Perspectives of microbial oils for biodiesel production. Appl Microbiol Biotechnol 80: 749-756 Li X, Hu H-Y, Gan K, Sun Y-X (2010) Effects of different nitrogen and phosphorus concentrations on the growth, nutrient uptake, and lipid accumulation of a freshwater microalga Scenedesmus sp. Bioresour Technol 101: 5494-5500 Li X, Xu H, Wu Q (2007) Large-scale biodiesel production from microalga Chlorella protothecoides through heterotrophic cultivation in bioreactors. Biotechnol Bioeng 98: 764-771 Li Y, Horsman M, Wang B, Wu N, Lan C (2008a) Effects of nitrogen sources on cell growth and lipid accumulation of green alga Neochloris oleoabundans. Appl Microbiol Biotechnol 81: 629-636 Li Y, Huang J, Sandmann G, Chen F (2008b) Glucose sensing and the mitochondrial alternative pathway are involved in the regulation of astaxanthin biosynthesis in the dark-grown Chlorella zofingiensis (Chlorophyceae). Planta 228: 735-743 Li Y, Huang J, Sandmann G, Chen F (2009) High-light and sodium chloride stress differently regulate the biosynthesis of astaxanthin in Chlorella zofingiensis (Chlorophyceae). J Phycol 45: 635-641 Liang Y, Sarkany N, Cui Y (2009) Biomass and lipid productivities of Chlorella vulgaris under autotrophic, heterotrophic and mixotrophic growth conditions. Biotechnol Lett 31: 1043-1049 Lim S, Teong LK (2010) Recent trends, opportunities and challenges of biodiesel 200 in Malaysia: An overview. Renew Sustain Energy Rev 14: 938-954 Linden H (1999) Carotenoid hydroxylase from Haematococcus pluvialis: cDNA sequence, regulation and functional complementation. Biochim Biophys Acta 1446: 203-212 Linden H, Misawa N, Sanito T, Sandmann G (1994) A novel carotenoid biosynthesis gene coding for zeta-carotene desaturase: functional expression, sequence and phylogenetic origin. Plant Mol Biol 24: 369-379 Linden H, Sandmann G, Chamovitz D, Hirschberg J, Boger P (1990) Generation and biochemical characterization of Synechococcus mutants selected against the bleaching herbicide norflurazon. Pest Biochem Physiol 36: 46-51 Liu C-Z, Wang F, Ou-Yang F (2009b) Ethanol fermentation in a magnetically fluidized bed reactor with immobilized Saccharomyces cerevisiae in magnetic particles. Bioresour Technol 100: 878-882 Liu X, Shibata T, Hisaka S, Osawa T (2009a) Astaxanthin inhibits reactive oxygen species-mediated cellular toxicity in dopaminergic SH-SY5Y cells via mitochondria-targeted protective mechanism. Brain Res 1254: 18-27 Liu X-J, Jiang Y, Chen F (2005) Fatty acid profile of the edible filamentous cyanobacterium Nostoc flagelliforme at different temperatures and developmental stages in liquid suspension culture. Process Biochem 40: 371-377 Liu YS, Wu JY (2007) Optimization of cell growth and carotenoid production of Xanthophyllomyces dendrorhous through statistical experiment design. Biochem Eng J 36: 182-189 Liu Z-Y, Wang G-C, Zhou B-C (2008) Effect of iron on growth and lipid accumulation in Chlorella vulgaris. Bioresour Technol 99: 4717-4722 Lockwood SF, Penn MS, Hazen SL, Bikadi Z, Zsila F (2006) The effects of oral Cardax(TM) (disodium disuccinate astaxanthin) on multiple independent oxidative stress markers in a mouse peritoneal inflammation model: influence on 5-lipoxygenase in vitro and in vivo. Life Sci 79: 162-174 Lohr M, Im C-S, Grossman AR (2005) Genome-based examination of chlorophyll 201 and carotenoid biosynthesis in Chlamydomonas reinhardtii. Plant Physiol 138: 490-515 Lorenz RT, Cysewski GR (2000) Commercial potential for Haematococcus microalgae as a natural source of astaxanthin. Trends Biotechnol 18: 160-167 Lynch DV, Thompson GA, Jr. (1982) Low temperature-induced alterations in the chloroplast and microsomal membranes of Dunaliella salina. Plant Physiol 69: 1369-1375 Lyons NM, O'Brien NM (2002) Modulatory effects of an algal extract containing astaxanthin on UVA-irradiated cells in culture. J Dermatol Sci 30: 73-84 Ma F, Hanna MA (1999) Biodiesel production: a review. Bioresour Technol 70: 1-15 Ma Y (2001) Physiological response of Chlorococcum sp. to external stresses. The University of Hong Kong, Hong Kong Mandeville S, Yaylayan V, Simpson B, Ramaswamy H (1991) Isolation and identification of carotenoid pigments, lipids and flavor active components from raw commercial shrimp waste. Food Biotechnol 5: 185-195 Mann V, Harker M, Pecker I, Hirschberg J (2000) Metabolic engineering of astaxanthin production in tobacco flowers. Nat Biotechnol 18: 888-892 Mansour MP, Volkman JK, Jackson AE, Blackburn SI (1999) The fatty acid and sterol composition of five marine dinoflagellates. J Phycol 35: 710-720 Martinez-ferez IM, Vioque A (1992) Nucleotide-sequence of the phytoene desaturase gene from Synechocystis sp. PCC 6803 and characterization of a new mutation which confers resistance to the herbicide norflurazon. Plant Mol Biol 18: 981-983 Mata TM, Martins AA, Caetano NS (2010) Microalgae for biodiesel production and other applications: A review. Renew Sustain Energy Rev 14: 217-232 McCarthy SS, Kobayashi MC, Niyogi KK (2004) White mutants of Chlamydomonas reinhardtii are defective in phytoene synthase. Genetics 168: 1249-1257 McNulty HP, Byun J, Lockwood SF, Jacob RF, Mason RP (2007) Differential 202 effects of carotenoids on lipid peroxidation due to membrane interactions: X-ray diffraction analysis. Biochim Biophys Acta 1768: 167-174 Meher LC, Naik SN, Naik MK, Dalai AK (2008) Biodiesel production using karanja (Pongamia pinnata) and jatropha (Jatropha curcas) seed oil. In: Pandey A (ed) Handbook of plant-based biofuels. CRC Press, Boca Raton, FL pp 255-266 Miao F, Lu D, Li Y, Zeng M (2006) Characterization of astaxanthin esters in Haematococcus pluvialis by liquid chromatography-atmospheric pressure chemical ionization mass spectrometry. Anal Biochem 352: 176-181 Miao X, Li R, Yao H (2009) Effective acid-catalyzed transesterification for biodiesel production. Energ Convers Manage 50: 2680-2684 Miao X, Wu Q (2004) High yield bio-oil production from fast pyrolysis by metabolic controlling of Chlorella protothecoides. J Biotechnol 110: 85-93 Miao X, Wu Q (2006) Biodiesel production from heterotrophic microalgal oil. Bioresour Technol 97: 841-846 Michel A, Arias RS, Scheffler BE, Duke SO, Netherland M, Dayan FE (2004) Somatic mutation-mediated evolution of herbicide resistance in the nonindigenous invasive plant hydrilla (Hydrilla verticillata). Mol Ecol 13: 3229-3237 Mike W, Hosoda K, Kondo K, Itakura H (1998) Astaxanthin-containing drink. Japanese patent #10155459 Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31: 426-429 Misawa N, Nakagawa M, Kobayashi K, Yamano S, Izawa Y, Nakamura K, Harashima K (1990) Elucidation of the Erwinia uredovora carotenoid biosynthetic pathway by functional analysis of gene products expressed in Escherichia coli. J Bacteriol 172: 6704-6712 Misawa N, Satomi Y, Kondo K, Yokoyama A, Kajiwara S, Saito T, Ohtani T, Miki W (1995) Structure and functional analysis of a marine bacterial carotenoid biosynthesis gene-cluster and astaxanthin biosynthetic pathway 203 proposed at the gene level. J Bacteriol 177: 6575-6584 Misawa N, Yamano S, Linden H, Defelipe MR, Lucas M, Ikenaga H, Sandmann G (1993) Functional expression of the Erwinia uredovora carotenoid biosynthesis gene crtl in transgenic plants showing an increase of beta-carotene biosynthesis activity and resistance to the beaching herbicide norflurazon. Plant J 4: 833-840 Moller IM (2001) Plant mitochondria and oxidative stress: electron transport, NADPH turnover, and metabolism of reactive oxygen species. Annu Rev Plant Phys 52: 561-591 Moore B, Zhou L, Rolland F, Hall Q, Cheng W-H, Liu Y-X, Hwang I, Jones T, Sheen J (2003) Role of the Arabidopsis glucose sensor HXK1 in nutrient, light, and hormonal signaling. Science 300: 332-336 Morris WL, Ducreux LJM, Fraser PD, Millam S, Taylor MA (2006) Engineering ketocarotenoid biosynthesis in potato tubers. Metab Eng 8: 253-263 Muller FL, Lustgarten MS, Jang Y, Richardson A, Van Remmen H (2007) Trends in oxidative aging theories. Free Radical Bio Med 43: 477-503 Munoz R, Guieysse B (2006) Algal-bacterial processes for the treatment of hazardous contaminants: A review. Water Res 40: 2799-2815 Naguib YMA (2000) Antioxidant activities of astaxanthin and related carotenoids. J Agr Food Chem 48: 1150-1154 Najafpour GD, Shan CP (2003) Enzymatic hydrolysis of molasses. Bioresour Technol 86: 91-94 Nakpong P, Wootthikanokkhan S (2010) High free fatty acid coconut oil as a potential feedstock for biodiesel production in Thailand. Renew Energy 35: 1682-1686 Napolitano GE (1994) The relationship of lipids with light and chlorophyll measurements in freshwater algae and periphyton. J Phycol 30: 943-950 Niamnuy C, Devahastin S, Soponronnarit S, Vijaya Raghavan GS (2008) Kinetics of astaxanthin degradation and color changes of dried shrimp during storage. J Food Eng 87: 591-600 204 Nichols BW (1965) Light induced changes in the lipids of Chlorella vulgaris. Biochim Biophysi Acta 106: 274-279 Niu J, Tian L-X, Liu Y-J, Yang H-J, Ye C-X, Gao W, Mai K-S (2009) Effect of dietary astaxanthin on growth, survival, and stress tolerance of postlarval shrimp, Litopenaeus vannamei. J World Aquaculture Soc 40: 795-802 Ohlrogge JB, Jaworski JG (1997) Regulation of fatty acid synthesis. Annu Rev Plant Phys 48: 109-136 Ohresser M, Matagne RF, Loppes R (1997) Expression of the arylsulphatase reporter gene under the control of the nit1 promoter in Chlamydomonas reinhardtii. Curr Genet 31: 264-271 Olaizola M (2000) Commercial production of astaxanthin from Haematococcus pluvialis using 25,000-liter outdoor photobioreactors. J Appl Phycol 12: 499-506 Olsen RL, Jacobsen T (1995) Characterization of flash-dried shrimp processing waste. J Mar Biotechnol 3: 208-209 Oner C, Altun S (2009) Biodiesel production from inedible animal tallow and an experimental investigation of its use as alternative fuel in a direct injection diesel engine. Appl Energ 86: 2114-2120 Orcutt DM, Patterson GW (1975) Sterol, fatty acid and elemental composition of diatoms grown in chemically defined media. Comp Biochem Physiol 50: 579-583 Orosa M, Torres E, Fidalgo P, Abalde J (2000) Production and analysis of secondary carotenoids in green algae. J Appl Phycol 12: 553-556 Orosa M, Valero JF, Herrero C, Abalde J (2001) Comparison of the accumulation of astaxanthin in Haematococcus pluvialis and other green microalgae under N-starvation and high light conditions. Biotechnol Lett 23: 1079-1085 Osterlie M, Bjerkeng B, Liaaen-Jensen S (1999) Accumulation of astaxanthin all-E, 9Z and 13Z geometrical isomers and 3 and 3' RS optical isomers in rainbow trout (Oncorhynchus mykiss) is selective. J Nutr 129: 391-398 Otsuka H (1960) Changes of lipid and carbohydrate contents of Chlorella cells 205 during the sulfur starvatoin, as studied by the technique of synchronous culture. J Gen App Microbiol 7: 72-77 Palozza P, Krinsky NI (1992) Astaxanthin and canthaxanthin are potent antioxidants in a membrane model. Arch Biochem Biophys 297: 291-295 Papas AM (1999) Antioxidant status, diet, nutrition, and health. CRC Press Parajo JC, Santos V, Vazquez M (1998) Optimization of carotenoid production by Phaffia rhodozyma cells grown on xylose. Process Biochem 33: 181-187 Parida AK, Das AB (2005) Salt tolerance and salinity effects on plants: a review. Ecotoxicol Environ Saf 60: 324-349 Pashkow FJ, Watumull DG, Campbell CL (2008) Astaxanthin: A novel potential treatment for oxidative stress and inflammation in cardiovascular disease. Am J Cardiol 101: S58-S68 Pecker I, Chamovitz D, Linden H, Sandmann G, Hirschberg J (1992) A single polypeptide catalyzing the conversion of phytoene to zeta-carotene is transcriptionally regulated during tomato fruit ripening. Proc Natl Acad Sci USA 89: 4962-4966 Peng J, Xiang W, Tang Q, Sun N, Chen F, Yuan J (2008) Comparative analysis of astaxanthin and its esters in the mutant E1 of Haematococcus pluvialis and other green algae by HPLC with a C30 column. Sci China C Life Sci 51: 1108-1115 Phan AN, Phan TM (2008) Biodiesel production from waste cooking oils. Fuel 87: 3490-3496 Pickett-Heaps JD (1975) Green algae: structure, reproduction and evolution in selected genera. Sinauer Associates, Sunderland Piorreck M, Pohl P (1984) Preparatory experiments for the axenic mass-culture of microalgae. 2. Formation of biomass, total protein, chlorophylls, lipids and fatty-acids in green and blue green-algae during one growth-phase. Phytochem 23: 217-223 Poulsen N, Kroger N (2005) A new molecular tool for transgenic diatomsControl of mRNA and protein 206 biosynthesis by an inducible promoter-terminator cassette. FEBS J 272: 3413-3423 Pruvost J, Van Vooren G, Cogne G, Legrand J (2009) Investigation of biomass and lipids production with Neochloris oleoabundans in photobioreactor. Bioresour Technol 100: 5988-5995 Pulz O, Gross W (2004) Valuable products from biotechnology of microalgae. Appl Microbiol Biotechnol 65: 635-648 Pushparaj B, Buccioni A, Paperi R, Piccardi R, Ena A, Carlozzi P, Sili C (2008) Fatty acid composition of Antarctic cyanobacteria. Phycologia 47: 430-434 Qian J, Wang F, Liu S, Yun Z (2008) In situ alkaline transesterification of cottonseed oil for production of biodiesel and nontoxic cottonseed meal. Bioresour Technol 99: 9009-9012 Quesada-Chanto A, Afschar AS, Wagner F (1994) Microbial production of propionic acid and vitamin B12 using molasses or sugar. Appl Microbiol Biotechnol 41: 378-383 Rabbani S, Beyer P, Lintig Jv, Hugueney P, Kleinig H (1998) Induced beta-carotene synthesis driven by triacylglycerol deposition in the unicellular alga Dunaliella bardawil. Plant Physiol 116: 1239-1248 Rada B, Leto T (2008) Oxidative innate immune defenses by Nox/Duox family NADPH oxidases. In: Egesten A, Schmidt A, Herwald H (eds) Trends in innate immunity. Karger, Basel, pp 164-197 Radmer RJ (1996) Algal diversity and commercial algal products. BioScience 46: 263-270 Raita M, Champreda V, Laosiripojana N (2010) Biocatalytic ethanolysis of palm oil for biodiesel production using microcrystalline lipase in tert-butanol system. Process Biochem 45: 829-834 Ralley L, Enfissi EMA, Misawa N, Schuch W, Bramley PM, Fraser PD (2004) Metabolic engineering of ketocarotenoid formation in higher plants. Plant J 39: 477-486 Ramirez J, Gutierrez H, Gschaedler A (2001) Optimization of astaxanthin production by Phaffia rhodozyma through factorial design and response 207 surface methodology. J Biotechnol 88: 259-268 Ramirez J, Nunez ML, Valdivia R (2000) Increased astaxanthin production by a Phaffia rhodozyma mutant grown on date juice from Yucca fillifera. J Ind Microbiol Biotechnol 24: 187-190 Ramos A, Coesel S, Marques A, Rodrigues M, Baumgartner A, Noronha J, Rauter A, Brenig B, Varela J (2008) Isolation and characterization of a stress-inducible Dunaliella salina Lcy-β gene encoding a functional lycopene β-cyclase. Appl Microbiol Biotechnol 79: 819-828 Randolph-Anderson BL, Sato R, Johnson AM, Harris EH, Hauser CR, Oeda K, Ishige F, Nishio S, Gillham NW, Boynton JE (1998) Isolation and characterization of a mutant protoporphyrinogen oxidase gene from Chlamydomonas reinhardtii conferring resistance to porphyric herbicides. Plant Mol Biol 38: 839-859 Ranganathan SV, Narasimhan SL, Muthukumar K (2008) An overview of enzymatic production of biodiesel. Bioresour Technol 99: 3975-3981 Rao AR, Dayananda C, Sarada R, Shamala TR, Ravishankar GA (2007) Effect of salinity on growth of green alga Botryococcus braunii and its constituents. Bioresour Technol 98: 560-564 Rashid U, Anwar F (2008) Production of biodiesel through optimized alkaline-catalyzed transesterification of rapeseed oil. Fuel 87: 265-273 Reitan KI, Rainuzzo JR, Olsen Y (1994) Effect of nutrient limitation on fatty acid and lipid content of marine microalgae. J Phycol 30: 972-979 REN21 (2009) Renewables global status report: 2009 update. http://www.ren21.net/pdf/RE_GSR_2009_update.pdf Renaud SM, Thinh L-V, Lambrinidis G, Parry DL (2002) Effect of temperature on growth, chemical composition and fatty acid composition of tropical Australian microalgae grown in batch cultures. Aquaculture 211: 195-214 Renaud SM, Zhou HC, Parry DL, Thinh L-V, Woo KC (1995) Effect of temperature on the growth, total lipid content and fatty acid composition of recently isolated tropical microalgae Isochrysis sp., Nitzschia closterium, 208 Nitzschia paleacea, and commercial species Isochrysis sp. (clone T.ISO). J Appl Phycol 7: 595-602 Rise M, Cohen E, Vishkautsan M, Cojocaru M, Gottlieb HE, Arad SM (1994) Accumulation of secondary carotenoids in Chlorella zofingiensis. J Plant Physiol 144: 287-292 Rodolfi L, Zittelli GC, Niccol?Bassi, Padovani G, Biondi N, Bonini G, Tredici MR (2009) Microalgae for oil: Strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol Bioeng 102: 100-112 Roesler K, Shintani D, Savage L, Boddupalli S, Ohlrogge J (1997) Targeting of the Arabidopsis homomeric acetyl-coenzyme A carboxylase to plastids of rapeseeds. Plant Physiol 113: 75-81 Roessler PG (1988) Changes in the activities of various lipid and carbohydrate biosynthetic enzymes in the diatom Cyclotella cryptica in response to silicon deficiency. Arch Biochem Biophys 267: 521-528 Roessler PG, Bleibaum JL, Thompson GA, Ohlrogge JB (1994) Characteristics of the gene that encodes acetyl-CoA carboxylase in the diatom Cyclotella cryptica. Anna NY Acad Sci 721: 250-256 Roessler PG, Ohlrogge JB (1993) Cloning and characterization of the gene that encodes acetyl-coenzyme A carboxylase in the alga Cyclotella cryptica. J Biol Chem 268: 19254-19259 Rolland F, Baena-Gonzalez E, Sheen J (2006) Sugar sensing and signaling in plants: Conserved and novel mechanisms. Ann Rev Plant Biol 57: 675-709 Romer S, Fraser PD, Kiano JW, Shipton CA, Misawa N, Schuch W, Bramley PM (2000) Elevation of the provitamin A content of transgenic tomato plants. Nat Biotechnol 18: 666-669 Ronen G, Carmel-Goren L, Zamir D, Hirschberg J (2000) An alternative pathway to β-carotene formation in plant chromoplasts discovered by map-based cloning of Beta and old-gold color mutations in tomato. Proc Natl Acad Sci USA 97: 11102-11107 209 Ronen G, Cohen M, Zamir D, Hirschberg J (1999) Regulation of carotenoid biosynthesis during tomato fruit development: expression of the gene for lycopene epsilon-cyclase is down-regulated during ripening and is elevated in the mutant Delta. Plant J 17: 341-351 Ross AB, Jones JM, Kubacki ML, Bridgeman T (2008) Classification of macroalgae as fuel and its thermochemical behaviour. Bioresour Technol 99: 6494-6504 Roukas T (1998) Pretreatment of beet molasses to increase pullulan production. Process Biochem 33: 805-810 Ryu JY, Song JY, Lee JM, Jeong SW, Chow WS, Choi SB, Pogson BJ, Park YI (2004) Glucose-induced expression of carotenoid biosynthesis genes in the dark is mediated by cytosolic pH in the cyanobacterium Synechocystis sp PCC 6803. J Biol Chem 279: 25320-25325 Sachindra NM, Bhaskar N, Siddegowda GS, Sathisha AD, Suresh PV (2007) Recovery of carotenoids from ensilaged shrimp waste. Bioresour Technol 98: 1642-1646 Sachindra NM, Mahendrakar NS (2005) Process optimization for extraction of carotenoids from shrimp waste with vegetable oils. Bioresour Technol 96: 1195-1200 Saha SK, Uma L, Subramanian G (2003) Nitrogen stress induced changes in the marine cyanobacterium Oscillatoria willei BDU 130511. FEMS Microbiol Ecol 45: 263-272 Sahoo PK, Das LM (2009) Process optimization for biodiesel production from Jatropha, Karanja and Polanga oils. Fuel 88: 1588-1594 Saka S, Kusdiana D (2001) Biodiesel fuel from rapeseed oil as prepared in supercritical methanol. Fuel 80: 225-231 Sakamoto T, Wada H, Nishida I, Ohmori M, Murata N (1994) delta 9 Acyl-lipid desaturases of cyanobacteria: molecular cloning and substrate specificities in terms of fatty acids, sn-positions, and polar head groups. J Biol Chem 269: 25576-25580 210 Sandesh Kamath B, Vidhyavathi R, Sarada R, Ravishankar GA (2008) Enhancement of carotenoids by mutation and stress induced carotenogenic genes in Haematococcus pluvialis mutants. Bioresour Technol 99: 8667-8673 Sandmann G (1994) Carotenoid biosynthesis in microorganisms and plants. Eur J Biochem 223: 7-24 Sandmann G (2001) Carotenoid biosynthesis and biotechnological application. Arch Biochem Biophys 385: 4-12 Sandmann G (2002) Molecular evolution of carotenoid biosynthesis from bacteria to plants. Physiol Plant 116: 431-440 Sandmann G, Kuhn M, Boger P (1993) Carotenoids in photosynthesis: protection of D1 degradation in the light. Photosynth Res 35: 185-190 Sandmann G, Linden H, Boger P (1989) Enzyme-kinetic studies on the interaction of norflurazon with phytoene desaturase. Z Naturforsch C 44: 787-790 Santocono M, Zurria M, Berrettini M, Fedeli D, Falcioni G (2006) Influence of astaxanthin, zeaxanthin and lutein on DNA damage and repair in UVA-irradiated cells. J Photochem Photobiol B 85: 205-215 Santocono M, Zurria M, Berrettini M, Fedeli D, Falcioni G (2007) Lutein, zeaxanthin and astaxanthin protect against DNA damage in SK-N-SH human neuroblastoma cells induced by reactive nitrogen species. J Photochem Photobiol B 88: 1-10 Sato N, Hagio M, Wada H, Tsuzuki M (2000) Environmental effects on acidic lipids of thylakoid membranes. In: Harwood JL, Quinn PJ (eds) Recent advances in biochemistry of plant lipids. Portland Press, London, pp 912-914 Sato N, Murata N (1980) Temperature shift-induced responses in lipids in the blue-green alga, Anabaena variabilis: The central role of diacylmonogalactosylglycerol in thermo-adaptation. Biochim Biophys Acta 619: 353-366 Schmitt R, Fabry S, Kirk DL (1992) In search of molecular origins of cellular differentiation in Volvox and its relatives. Intl Rev Cytol 139: 189-256 211 Schroda M, Blocker D, Beck CF (2000) The HSP70A promoter as a tool for the improved expression of transgenes in Chlamydomonas. Plant J 21: 121-131 Scolnik PA, Bartley GE (1993) Phytoene desaturase from Arabidopsis. Plant Physiol 103: 1475 Seybold A, Goodwin T (1959) Occurrence of astaxanthin in the flower petals in Adonis annua L. Nature 184: 1714-1715 Shafiee S, Topal E (2009) When will fossil fuel reserves be diminished? Energy Policy 37: 181-189 Shah S, Gupta MN (2007) Lipase catalyzed preparation of biodiesel from Jatropha oil in a solvent free system. Process Biochem 42: 409-414 Shahidi F, Synowiecki J (1991) Isolation and characterization of nutrients and value-added products from snow crab (Chionoecetes opilio) and shrimp (Pandalus borealis) processing discards. J Agr Food Chem 39: 1527-1532 Sharma A, Vivekanand V, Singh RP (2008) Solid-state fermentation for gluconic acid production from sugarcane molasses by Aspergillus niger ARNU-4 employing tea waste as the novel solid support. Bioresour Technol 99: 3444-3450 Shay EG (1993) Diesel fuel from vegetable oils: status and opportunities. Biom Bioenerg 4: 227-242 Sheehan J, Dunahay T, Benemann J, Roessler P (1998) A look back at the U.S. Department of Energy's aquatic species programme - Biodiesel from algae. Report No. NREL/TP-580-24190; National Renewable Energy Laboratory: Golden, CO Shewmaker CK, Sheehy JA, Daley M, Colburn S, Ke DY (1999) Seed-specific overexpression of phytoene synthase: increase in carotenoids and other metabolic effects. Plant J 20: 401-412 Shi X-M, Chen F (2002) High-yield production of lutein by the green microalga Chlorella protothecoides in heterotrophic fed-batch culture. Biotechnol Progr 18: 723-727 Shimidzu N, Goto M, Miki W (1996) Carotenoids as singlet oxygen quenchers in 212 marine organisms. Fisheries Sci 62: 134-137 Singh SP, Singh D (2010) Biodiesel production through the use of different sources and characterization of oils and their esters as the substitute of diesel: A review. Renew Sustain Energy Rev 14: 200-216 Singhania RR, Parameswaran B, Pandey A (2008) Plant-based bioufuels: an introduction. In: Pandey A (ed) Handbook of plant-based biofuels. CRC Press, Boca Raton, FL, pp 3-12 Sobrado P, Lyle KS, Kaul SP, Turco MM, Arabshahi I, Marwah A, Fox BG (2006) Identification of the binding region of the [2Fe-2S] ferredoxin in stearoyl-acyl carrier protein desaturase: insight into the catalytic complex and mechanism of action. Biochem 45: 4848-4858 Solovchenko A, Khozin-Goldberg I, Didi-Cohen S, Cohen Z, Merzlyak M (2008) Effects of light intensity and nitrogen starvation on growth, total fatty acids and arachidonic acid in the green microalga Parietochloris incisa. J Appl Phycol 20: 245-251 Somerville C (1995) Direct tests of the role of membrane lipid composition in low-temperature-induced photoinhibition and chilling sensitivity in plants and cyanobacteria. Proc Natl Acad Sci USA 92: 6215-6218 Spolaore P, Joannis-Cassan C, Duran E, Isambert A (2006) Commercial applications of microalgae. J Biosci Bioeng 101: 87-96 Stadtman E (1992) Protein oxidation and aging. Science 257: 1220-1224 Stalberg K, Lindgren O, Ek B, Hoglund AS (2003) Synthesis of ketocarotenoids in the seed of Arabidopsis thaliana. Plant J 36: 771-779 Steinbrenner J, Linden H (2001) Regulation of two carotenoid biosynthesis genes coding for phytoene synthase and carotenoid hydroxylase during stress-induced astaxanthin formation in the green alga Haematococcus pluvialis. Plant Physiol 125: 810-817 Steinbrenner J, Linden H (2003) Light induction of carotenoid biosynthesis genes in the green alga Haematococcus pluvialis: regulation by photosynthetic redox control. Plant Mol Biol 52: 343-356 213 Steinbrenner J, Sandmann G (2006) Transformation of the green alga Haematococcus pluvialis with a phytoene desaturase for accelerated astaxanthin biosynthesis. Appl Environ Microb 72: 7477-7484 Stewart CN, Via LE (1993) A rapid CTAB DNA isolation technique useful for rapid fingerprinting and other PCR applications. Biotechniques 14: 748-751 Storebakken T, Goswami UC (1996) Plasma carotenoid concentration indicates the availability of dietary astaxanthin for Atlantic salmon, Salmo salar. Aquaculture 146: 147-153 Storebakken T, No HK (1992) Pigmentation of rainbow trout. Aquaculture 100: 209-229 Storebakken T, Sorensen M, Bjerkeng B, Hiu S (2004) Utilization of astaxanthin from red yeast, Xanthophyllomyces dendrorhous, in rainbow trout, Oncorhynchus mykiss: effects of enzymatic cell wall disruption and feed extrusion temperature. Aquaculture 236: 391-403 Su E-Z, Zhang M-J, Zhang J-G, Gao J-F, Wei D-Z (2007) Lipase-catalyzed irreversible transesterification of vegetable oils for fatty acid methyl esters production with dimethyl carbonate as the acyl acceptor. Biochem Eng J 36: 167-173 Sukenik A (1999) Production of eicosapentaenoic acid by the marine eustigmatophyte Nannochloropsis. In: Cohen Z (ed) Chemicals from microalgae. Taylor & Francis, London, pp 41-56 Sukenik A, Carmeli Y, Berner T (1989) Regulation of fatty acid composition by irradiance level in the eustigmatophyte Nannochloropsis sp. J Phycol 25: 686-692 Sun N (2009) High yield production of astaxanthin by Chlorella zofingiensis (Chlorophyta). Chinese Acedemy of Sciences, Guangzhou, China Sun N, Wang Y, Li Y-T, Huang J-C, Chen F (2008) Sugar-based growth, astaxanthin accumulation and carotenogenic transcription of heterotrophic Chlorella zofingiensis (Chlorophyta). Process Biochem 43: 1288-1292 Sun Z, Gantt E, Cunningham FXJ (1996) Cloning and functional analysis of the 214 β-carotene hydroxylase of Arabidopsis thaliana. J Biol Chem 271: 24349-24352 Suresh B, Ravishankar GA (2004) Phytoremediation - A novel and promising approach for environmental clean-up. Crit Rev Biotechnol 24: 97-124 Takagi M, Karseno, Yoshida T (2006) Effect of salt concentration on intracellular accumulation of lipids and triacylglyceride in marine microalgae Dunaliella cells. J Biosci Bioeng 101: 223-226 Takagi M, Watanabe K, Yamaberi K, Yoshida T (2000) Limited feeding of potassium nitrate for intracellular lipid and triglyceride accumulation of Nannochloris sp. UTEX LB1999. Appl Microbiol Biotechnol 54: 112-117 Tanaka T, Makita H, Ohnishi M, Mori H, Satoh K, Hara A (1995) Chemoprevention of rat oral carcinogenesis by naturally-occurring xanthophylls, astaxanthin and canthaxarathin. Cancer Res 55: 4059-4064 Tanaka T, Morishita Y, Suzui M, Kojima T, Okumura A, Mori H (1994) Chemoprevention of mouse urinary-bladder carcinogenesis by the naturally-occurring carotenoid astaxanthin. Carcinogenesis 15: 15-19 Tatsuzawa H, Takizawa E, Wada M, Yamamoto Y (1996) Fatty acid and lipid composition of the acidophilic green alga Chlamydomonas sp. J Phycol 32: 598-601 Thomas D (2002) Seaweeds. Natural History Museum, London Thompson PA, Guo M-x, Harrison PJ, Whyte JNC (1992) Effects of variation in temperature 2. On the fatty acid composition of 8 species of marine phytoplankton. J Phycol 28: 488-497 Thompson W, Meyer S, Green T (2010) The U.S. biodiesel use mandate and biodiesel feedstock markets. Biom Bioenerg 34: 883-889 Thurmond W (2008) Biodiesel 2020: a global market survey. Emerging Markets Online. http://www.emerging-markets.com/biodiesel/ Tjahjono AE, Kakizono T, Hayama Y, Nishio N, Nagai S (1994) Isolation of resistant mutants against carotenoid biosynthesis inhibitors for a green alga Haematococcus pluvialis, and their hybrid formation by protoplast fusion for 215 breeding of higher astaxanthin producers. J Ferment Bioeng 77: 352-357 Torres CF, Lin B, Lessard LP, Hill JCG (2005) Lipase-mediated transesterification of menhaden oil with the ethyl ester of conjugated linoleic acid: multi-response kinetics. Biochem Eng J 23: 107-116 Torrisen OJ (1986) Pigmentation of salmonids - A comparison of astaxanthin and canthaxanthin as pigment sources for rainbow trout. Aquaculture 53: 271-278 Tripathi DN, Jena GB (2009) Intervention of astaxanthin against cyclophosphamide-induced oxidative stress and DNA damage: A study in mice. Chem-Biol Interact 180: 398-406 Tripathi U, Venkateshwaran G, Sarada R, Ravishankar GA (2001) Studies on Haematococcus pluvialis for improved production of astaxanthin by mutagenesis. World J Microbiol Biotechnol 17: 143-148 Turujman SA, Wamer WG, Wei RR, Albert RH (1997) Rapid liquid chromatographic method to distinguish wild salmon from aquacultured salmon fed synthetic astaxanthin. J Aoac Int 80: 622-632 Ukibe K, Katsuragi T, Tani Y, Takagi H (2008) Efficient screening for astaxanthin-overproducing mutants of the yeast Xanthophyllomyces dendrorhous by flow cytometry. FEMS Microbiol Lett 286: 241-248 Vasudevan P, Briggs M (2008) Biodiesel production - current state of the art and challenges. J Ind Microbiol Biotechnol 35: 421-430 Visser H, van Ooyen AJJ, Verdoes JC (2003) Metabolic engineering of the astaxanthin-biosynthetic pathway of Xanthophyllomyces dendrorhous. FEMS Yeast Res 4: 221-231 Wada H, Murata N (1990) Temperature-induced changes in the fatty acid composition of the cyanobacterium, Synechocystis PCC6803. Plant Physiol 92: 1062-1069 Wade N, Goulter KC, Wilson KJ, Hall MR, Degnan BM (2005) Esterified astaxanthin levels in lobster epithelia correlate with shell colour intensity: Potential role in crustacean shell colour formation. Comp Biochem Phsy B 216 141: 307-313 Wang Y, Chen T (2008) The biosynthetic pathway of carotenoids in the astaxanthin-producing green alga Chlorella zofingiensis. World J Microbiol Biotechnol 24: 2927-2932 Wen ZY, Chen F (2001) Optimization of nitrogen sources for heterotrophic production of eicosapentaenoic acid by the diatom Nitzschia laevis. Enzyme Microb Tech 29: 341-347 Wen ZY, Chen F (2003) Heterotrophic production of eicosapentaenoic acid by microalgae. Biotechnol Adv 21: 273-294 Wikipedia (2010) Green algae. http://en.wikipedia.org/wiki/Green_algae Williams KC (2007) Nutritional requirements and feeds development for post-larval spiny lobster: A review. Aquaculture 263: 1-14 Wolf AM, Asoh S, Hiranuma H, Ohsawa I, Iio K, Satou A, Ishikura M, Ohta S (2010) Astaxanthin protects mitochondrial redox state and functional integrity against oxidative stress. J Nutr Biochem 21: 381-389 Xiong W, Gao C, Yan D, Wu C, Wu Q (2010) Double CO2 fixation in photosynthesis-fermentation model enhances algal lipid synthesis for biodiesel production. Bioresour Technol 101: 2287-2293 Xu H, Miao X, Wu Q (2006) High quality biodiesel production from a microalga Chlorella protothecoides by heterotrophic growth in fermenters. J Biotechnol 126: 499-507 Ye XD, Al-Babili S, Kloti A, Zhang J, Lucca P, Beyer P, Potrykus I (2000) Engineering the provitamin A (beta-carotene) biosynthetic pathway into (carotenoid-free) rice endosperm. Science 287: 303-305 Yoo C, Jun S-Y, Lee J-Y, Ahn C-Y, Oh H-M (2009) Selection of microalgae for lipid production under high levels carbon dioxide. Bioresour Technol 101: S71-S74 Yoshida H, Yanai H, Ito K, Tomono Y, Koikeda T, Tsukahara H, Tada N (2010) Administration of natural astaxanthin increases serum HDL-cholesterol and adiponectin in subjects with mild hyperlipidemia. Atherosclerosis 209: 217 520-523 Zhang BY, Geng YH, Li ZK, Hu HJ, Li YG (2009) Production of astaxanthin from Haematococcus in open pond by two-stage growth one-step process. Aquaculture 295: 275-281 Zhang DH, Lee YK (1997) Enhanced accumulation of secondary carotenoids in a mutant of the green alga, Chlorococcum sp. J Appl Phycol 9: 459-463 Zhang Y, Dub MA, McLean DD, Kates M (2003) Biodiesel production from waste cooking oil: 1. Process design and technological assessment. Bioresour Technol 89: 1-16 Zhekisheva M, Boussiba S, Khozin-Goldberg I, Zarka A, Cohen Z (2002) Accumulation of oleic acid in Haematococcus pluvialis (Chlorophyceae) under nitrogen starvation or high light is correlated with that of astaxanthin esters. J Phycol 38: 325-331 Zhekisheva M, Zarka A, Khozin-Goldberg I, Cohen Z, Boussiba S (2005) Inhibition of astaxanthin synthesis under high irradiance does not abolish triacylglycerol accumulation in the green alga Haematococcus pluvialis (Chlorophyceae). J Phycol 41: 819-826 Zheng YG, Hu ZC, Wang Z, Shen YC (2006) Large-scale production of astaxanthin by Xanthophyllomyces dendrorhous. Food Bioprod Process 84: 164-166 Zhong Y, Huang J, Chen F (2008) Synthesis of astaxanthin in the leaves of Arabidopsis thaliana. J Biotechnol 136: S65-S65 Zhu YH, Jiang JG, Yan Y, Chen XW (2005) Isolation and characterization of phytoene desaturase cDNA involved in the beta-carotene biosynthetic pathway in Dunaliella salina. J Agr Food Chem 53: 5593-5597 218