From www.bloodjournal.org by guest on March 6, 2016. For personal use only.
TRANSPLANTATION
Brief report
Reproductive capability in dogs with canine leukocyte adhesion deficiency
treated with nonmyeloablative conditioning prior to allogeneic hematopoietic
stem cell transplantation
Tanya H. Burkholder, Lyn Colenda, Laura M. Tuschong, Matthew F. Starost, Thomas R. Bauer Jr, and Dennis D. Hickstein
Nonmyeloablative conditioning regimens
are increasingly replacing myeolablative
conditioning prior to allogeneic hematopoietic stem cell transplantation (SCT).
The recent advent of these conditioning
regimens has limited the assessment of
the long-term effects of this treatment,
including analysis of reproductive function. To address the question of reproductive function after nonmyeloablative trans-
plantation, we analyzed a cohort of young
dogs with the genetic disease canine
leukocyte adhesion deficiency that were
treated with a nonmyeloablative dose of
200 cGy total body irradiation followed by
matched-littermate SCT. Five males and
5 females entered puberty; all 5 males
and 4 females subsequently sired or delivered litters following transplantation. We
demonstrate that fertility is intact and
dogs have uncomplicated parturitions following nonmyeloablative conditioning for
SCT. These results are encouraging for
children and adults of childbearing age
who receive similar conditioning regimens prior to allogeneic transplantation.
(Blood. 2006;108:1767-1769)
© 2006 by The American Society of Hematology
Introduction
Hematopoietic stem cell transplantation (SCT) represents the only
definitive treatment for a number of hematologic diseases, including leukemia,1 hemoglobinopathies,2 and immunodeficiencies, such
as leukocyte adhesion deficiency.3 Due to the success of SCT many
patients are now living increased life spans free from their primary
disease. Thus, late complications of transplantation are increasing
in importance.
One complication of transplantation, reproductive failure, has a
high incidence and is a well-characterized late complication of
SCT in both children and adults.4 The conditioning regimens used
for SCT, including myeloablative dosages of chemotherapy and/or
total body irradiation (TBI), are responsible for many of the late
complications. In children, gonadal damage from irradiation can
result in delayed onset or absence of puberty, failure to achieve
menarche in girls, and infertility in both sexes.4,5 A large, retrospective survey of 19 412 allogeneic and 17 940 autologous transplantation patients showed an overall pregnancy rate of 0.6%, which was
10-fold lower than the crude birth rate for the control population.6
Recently, nonmyeloablative conditioning strategies have been
developed to reduce the complications of SCT and to extend the
possibility of transplantation to older individuals, those with
comorbid medical conditions, and children with nonmalignant
hematologic disease. Because of the limited period of observation
following nonmyeloablative transplantation in humans, the relationship of these reduced-intensity conditioning regimens to fertility
remains unclear.
The canine model is a well-recognized model for developing
new SCT regimens and for identifying complications following
SCT.7 We have described the successful use of matched-littermate
transplantation following nonmyeloablative conditioning with
200 cGy TBI in puppies with the immunodeficiency disease
canine leukocyte adhesion deficiency (CLAD).8,9 We now
describe the preservation of reproductive function and pregnancy outcomes in male and female puppies treated with this
nonmyeloablative conditioning regimen followed by matchedlittermate allogeneic transplantation.
From the Division of Veterinary Resources, Office of Research Services,
National Institutes of Health, Bethesda, MD; and the Experimental
Transplantation and Immunology Branch, Center for Cancer Research,
National Cancer Institute, National Institutes of Health, Bethesda, MD.
Research Services.
Submitted February 23, 2006; accepted April 15, 2006. Prepublished online
as Blood First Edition Paper, April 27, 2006; DOI 10.1182/blood-200602-005645.
Supported by the Intramural Research Program of the National Institutes of
Health, National Cancer Institute, Center for Cancer Research and Office of
BLOOD, 1 SEPTEMBER 2006 䡠 VOLUME 108, NUMBER 5
Study design
Dogs
Animals in this study are all members of a breeding colony housed by the
Division of Veterinary Resources at the National Institutes of Health. All
procedures performed were approved by the Institutional Animal Care and
Use Committee of the National Cancer Institute.
Transplantation regimen
CLAD dogs were conditioned with TBI at a nonmyeloablative dose of
200 cGy delivered from a 60Co source on the day of transplantation. The
source of the hematopoietic stem cells, bone marrow cell infusion, and
posttransplantation immunosuppression have been described.9 All of the
dogs were treated before 4 months of age.
Reprints: Tanya H. Burkholder, Division of Veterinary Resources, Office of
Research Services, National Institutes of Health, 9000 Rockville Pike, Bldg 28,
Rm 104, Bethesda, MD 20892-0001; e-mail: burkholt@mail.nih.gov.
The publication costs of this article were defrayed in part by page charge
payment. Therefore, and solely to indicate this fact, this article is hereby
marked ‘‘advertisement’’ in accordance with 18 U.S.C. section 1734.
© 2006 by The American Society of Hematology
1767
From www.bloodjournal.org by guest on March 6, 2016. For personal use only.
1768
BLOOD, 1 SEPTEMBER 2006 䡠 VOLUME 108, NUMBER 5
BURKHOLDER et al
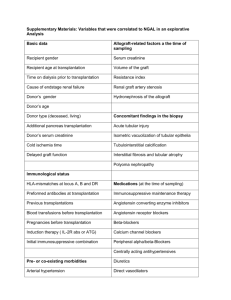
Table 1. Semen quality, paternity, and number of offspring produced by 5 male CLAD dogs after nonmyeloablative conditioning for SCT
Dog
Source of
HSCs
M1*
BM
M2
BM
M3
M4
M5
Time after
SCT, y
Semen analysis after SCT
Sperm count, ⴛ 106
Motility, %
1/4
1143/1348
70/90
87/92
2 (15)
2
High concentration
Good
High percentage
1 (5)
PBSCs
3
543
75
92
1 (6)
BM
3
585
90
93
2 (11)
CD34⫹ BM
3
High concentration
Good
High percentage
3 (28)
Morphology, % normal
Litters sired after SCT (no. puppies)
The conditioning regimen was 200 cGy TBI for each dog.
HSCs indicates hematopoietic stem cells; M, male; BM, bone marrow; PBSCs, peripheral-blood stem cells; BM CD34⫹, CD34⫹ fraction of bone marrow.
*Two sets of data are provided for this dog because semen was evaluated at 2 time points after SCT.
Fertility assessment and breeding
Estrus was detected by visual examination for vaginal discharge and
tumescence and confirmed with vaginal cytology prior to breeding. Dogs
were bred either by natural cover or by artificial insemination using fresh
semen on days 1, 3, and 5 of estrus. At the end of the study, all puppies with
birth defects and dogs that had received transplants were submitted to the
Pathology Section of the Division of Veterinary Resources for full gross and
histologic analysis. Semen was collected from conscious dogs and analyzed
using standard methods by an independent evaluator (Lee Jones, International Canine Semen Bank–Mobile Delaware, Hockessin, DE) experienced
in canine semen evaluation.
Results and discussion
Five male dogs in our study entered puberty and achieved
normal spermatogenesis by 1 year of age following nonmyeloblative conditioning with 200 cGy prior to SCT. This is the same
reproductive milestone seen in normal dogs not receiving
irradiation. All 5 have sired at least 1 litter (Table 1). Only 2 of
65 puppies from litters sired by the males that had received
transplants had congenital malformations. This incidence of
birth defects is not significantly different than the incidence of
1 of 69 puppies born to untreated carrier animals in our colony.
Five female dogs in our study had spontaneous onset of
estrus by 1 year of age. The average interestrus interval of 227
days was within normal limits for dogs (Table 2). Four of these
5 females subsequently delivered litters of healthy puppies by
natural parturition. Conception rates and litter sizes after SCT
treatment were similar to the rate observed in normal dogs. The
incidence of neonatal mortality in puppies born to females after
SCT was 3 (10%) of 30 puppies, which is similar to the
Table 2. Estrus cyclicity and parity in 5 female CLAD dogs after
nonmyeloablative conditioning for SCT
Dog
Source of
HSCs
Time after
SCT, y
Interestrus
interval, d
No. of
cycles
Pregnancies after
SCT (puppies)
1 (12)
F1*†
BM
3
249
4
F2†
BM
3
337
3
1 (7)
F3*
PBSCs
3
227
3
0
F4†
PBSCs
3
213
4
1 (11)
F5
BM
2
235
2
1 (6)
The conditioning regimen was 200 cGy TBI for each dog.
F indicates female; other abbreviations are explained in Table 1.
*F1 and F3 did not conceive or had an early loss of the pregnancy after their first
breeding. The sires used were a male conditioned with busulfan and a normal dog. F1
conceived on her second breeding and delivered a healthy litter. F3 has not
been rebred.
†Litters sired by males that had received transplants, producing 100% affected
progeny.
incidence of 5 (7%) of 77 puppies born to genetically similar
carrier animals in our colony. One (3%) of 30 puppies born to
females after SCT had a minor birth defect, which is similar to
the rate from untreated female dogs, supporting the findings of
others that conditioning, irrespective of regimen used or age of
the patient at the time of treatment, does not increase the
incidence of birth defects in the offspring.4,6
These results suggest that pregnancies in patients after
nonmyeloablative conditioning with 200 cGy TBI do not carry
the increased risk of complications that has previously been
reported for myeloablative conditioning in dogs and women.
The germinal epithelium of the testis is more vulnerable to
the effects of radiation-induced damage than either the ovary or
the Leydig cell.10 In 2 large studies, following conditioning that
includes TBI, most men failed to regain spermatogenesis,
although testosterone levels were generally normal.11,12 Several
studies have shown that following conditioning with TBI during
childhood, approximately two thirds of boys experienced germcell damage, although most had adequate testosterone concentrations and entered puberty spontaneously.5,13,14
In females, the age of the patient at the time of transplantation appears to be as important as the conditioning regimen in
predicting return of fertility. Twenty-one of 32 girls treated
prepubertally achieved menarche after conditioning with chemotherapy and a hyperfractionated TBI dose of either 13.75 or
15 Gy.5 The median age at transplantation for girls in this study
who did not achieve menarche was 8.6 years, versus 6.1 years
for those who achieved spontaneous puberty. These findings
suggest that in girls, the younger the age at transplantation the
greater the potential for normal ovarian function later in life,
which is likely because their ovaries contain a greater number of
oocytes.15 The treatment age of the puppies in our study is
analogous to very young prepubertal girls, which may have been
a positive contributing factor in their preservation of fertility.
While the ovary is less prone to damage during youth, the
growing uterus and uterine blood supply are more susceptible to
radiation-induced damaged resulting in a reduced uterine volume, a subsequent increased risk of miscarriage, and low-birthweight babies later in life.16 All of the pregnancies conceived by
5 women who were treated prepubertally with chemotherapy
and 10 to 15.75 Gy TBI resulted in spontaneous abortion.4 In
1 study of SCT in dogs, fertility was reported in 4 of 4 male and
5 of 7 female animals after treatment with 7.5 Gy TBI
administered at 8 to 18 months of age.17 However, 12 (55%) of
22 of the neonatal puppies died due to prolonged delivery times
as a result of secondary uterine inertia. From this study it was
not clear whether female dogs treated at a younger age
experienced a greater percentage of complications.
From www.bloodjournal.org by guest on March 6, 2016. For personal use only.
BLOOD, 1 SEPTEMBER 2006 䡠 VOLUME 108, NUMBER 5
The results from the canine model indicate that fertility is
retained following nonmyeloablative conditioning for SCT consisting of 200 cGy TBI and matched-littermate allogeneic transplantation in a genetic immunodeficiency disease. These results demonstrating intact fertility and uncomplicated parturitions after
nonmyeloablative conditioning and transplantation in dogs suggest
that children and adults of childbearing age receiving this regimen
might be expected to retain fertility as well during their subsequent
reproductive years.
FERTILITY AFTER BONE MARROW TRANSPLANTATION
1769
Acknowledgments
The authors thank Kevin Cogan for assistance collecting semen,
Anastasia Sowers and the Radiation Biology Branch of the
National Cancer Institute for performing TBI on the dogs, and the
veterinary care and enrichment staff of the Division of Veterinary
Resources for providing excellent care and training for the puppies
produced in this study.
References
1. Hayden PJ, Keogh F, Ni Conghaile M, et al. A
single-centre assessment of long-term qualityof-life status after sibling allogeneic stem cell
transplantation for chronic myeloid leukaemia in
first chronic phase. Bone Marrow Transplant.
2004;34:545-556.
2. Mentzer WC. Bone marrow transplantation for
hemoglobinopathies. Curr Opin Hematol. 2000;
7:95-100.
3. Thomas C, Le Deist F, Cavazzana-Calvo M, et al.
Results of allogeneic bone marrow transplantation in patients with leukocyte adhesion deficiency. Blood. 1995;86:1629-1635.
4. Sanders JE, Hawley J, Levy W, et al. Pregnancies following high-dose cyclophosphamide with
or without high-dose busulfan or total-body irradiation and bone marrow transplantation. Blood.
1996;87:3045-3052.
5. Sarafoglou K, Boulad F, Gillio A, Sklar C. Gonadal
function after bone marrow transplantation for
acute leukemia during childhood. J Pediatr. 1997;
130:210-216.
6. Salooja N, Szydlo RM, Socie G, et al. Pregnancy
outcomes after peripheral blood or bone marrow
transplantation: a retrospective study. Lancet.
2001;358:271-276.
7. Storb R, Kolb HJ, Deeg HJ, et al. Prevention of
graft-versus-host disease by immunosuppressive
agents after transplantation of DLA-nonidentical
canine marrow. Bone Marrow Transplant. 1986;1:
167-177.
8. Bauer TR Jr, Creevy KE, Gu YC, et al. Very low
levels of donor CD18⫹ neutrophils following allogeneic hematopoietic stem cell transplantation
reverse the disease phenotype in canine leukocyte adhesion deficiency. Blood. 2004;103:
3582-3589.
9. Bauer TR, Gu YC, Tuschong LM, et al. Nonmyeloablative hematopoietic stem cell transplantation corrects the disease phenotype in the canine
model of leukocyte adhesion deficiency. Exp Hematol. 2005;33:706-712.
10. Howell SJ, Shalet SM. Spermatogenesis after
cancer treatment: damage and recovery. J Nat
Cancer Inst Monogr. 2005;34:12-17.
11. Anserini P, Chiodi S, Spinelli S, et al. Semen
analysis following allogeneic bone marrow transplantation. Additional data for evidence-based
counseling. Bone Marrow Transplant. 2002;30:
447-451.
12. Sanders JE, Buckner CD, Leonard JM, et al. Late
effects of gonadal function of cyclophosphamide,
total-body irradiation, and marrow transplantation. Transplantation. 1983;36:252-255.
13. Couto-Silva AC, Trivin C, Thibaud E, Esperou
H, Michon J, Brauner R. Factors affecting gonadal function after bone marrow transplantation during childhood. Bone Marrow Transplant.
2001;28:67-75.
14. Sklar CA, Kim TH, Ramsay NK. Testicular function following bone marrow transplantation performed during or after puberty. Cancer. 1984;53:
1498-1501.
15. Wallace WH, Thomson AB, Kelsey TW. The radio
sensitivity of the human oocyte. Hum Reprod.
2003;18:117-121.
16. Bath LE, Critchley HO, Chambers SE, Anderson
RA, Kelnar CJ, Wallace WH. Ovarian and uterine
characteristics after total body irradiation in childhood and adolescence: response to sex steroid
replacement. Br J Obstet Gynaecol. 1999;106:
1265-1272.
17. Vriesendorp HM, Klapwyk WM, Heidt PJ, Hogeweg
B, Zurcher C, van Bekkum DW. Factors controlling
the engraftment of transplanted dog bone marrow
cells. Tissue Antigens. 1982;20:63-80.
From www.bloodjournal.org by guest on March 6, 2016. For personal use only.
2006 108: 1767-1769
doi:10.1182/blood-2006-02-005645 originally published
online April 27, 2006
Reproductive capability in dogs with canine leukocyte adhesion
deficiency treated with nonmyeloablative conditioning prior to
allogeneic hematopoietic stem cell transplantation
Tanya H. Burkholder, Lyn Colenda, Laura M. Tuschong, Matthew F. Starost, Thomas R. Bauer, Jr
and Dennis D. Hickstein
Updated information and services can be found at:
http://www.bloodjournal.org/content/108/5/1767.full.html
Articles on similar topics can be found in the following Blood collections
Brief Reports (1868 articles)
Cell Adhesion and Motility (790 articles)
Transplantation (2125 articles)
Information about reproducing this article in parts or in its entirety may be found online at:
http://www.bloodjournal.org/site/misc/rights.xhtml#repub_requests
Information about ordering reprints may be found online at:
http://www.bloodjournal.org/site/misc/rights.xhtml#reprints
Information about subscriptions and ASH membership may be found online at:
http://www.bloodjournal.org/site/subscriptions/index.xhtml
Blood (print ISSN 0006-4971, online ISSN 1528-0020), is published weekly by the American Society
of Hematology, 2021 L St, NW, Suite 900, Washington DC 20036.
Copyright 2011 by The American Society of Hematology; all rights reserved.