5.1. 12B. 1 - Warren Hills Regional School District
advertisement

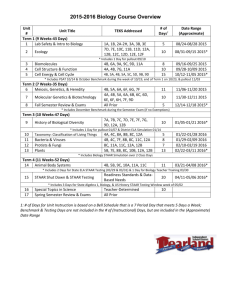
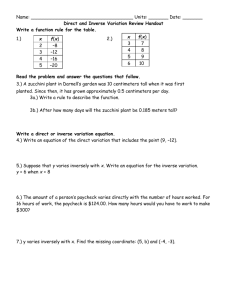
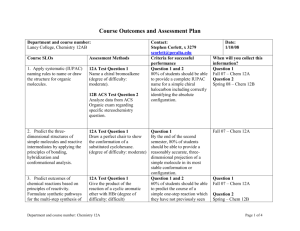
WARREN HILLS REGIONAL SCHOOLS: COURSE OF STUDY/CURRICULUM MAP Teacher:DeVivo/Luff/Hodge CONTENT Matter Chapter 1 Course: Technical Chemistry BENCHMARK 5.1. 12A. 3, 4 5.1. 12B. 1 5.1. 12C. 1 5.3. 12A. 1 5.3. 12B. 1 5.3. 12C. 1 5.6. 12A. 6 5.6. 12B. 1, 2 SKILLS Classify matter Compare metals and nonmetals ASSESSMENT Quiz *Vocabulary Ch 1 Review Physical and Chemical Properties and Changes Lab Test Significant Digits Conversions Problem-solving Techniques Chapter 2 12A. 12B. 12C. 12A. 12B. 3, 4 1, 2 1 1 1 Scientific Measurement Atoms Chapter 3 5.1. 5.1. 5.1. 5.3. 5.3. 5.1. 5.2. 5.2. 5.3. 5.6. 12C. 12A. 12B. 12A. 12A. 1 1 1, 2, 3 1 1, 2, 3 Determine significant digits Solve conversion problems Perform mathematical operations using rules for significant digits, rounding, and scientific notation Analyze error Perform lab using scientific measurements Assess the information on the periodic table Practice mole calculations Quiz *Vocabulary *Significant Digits *Math operations using significant digits Density Lab Ch 2 Review Test Quiz *Vocabulary *Mole calculations *Periodic Table Information (subatomic particles, atomic number, atomic mass, atomic weight) Ch 3 review TEST WARREN HILLS REGIONAL SCHOOLS: COURSE OF STUDY/CURRICULUM MAP Teacher:DeVivo/Luff/Hodge CONTENT Electron Configurations Hund’s Rule Chapter 4 Aufbau Principle Pauli Exclusion Principle Periodic Table Periodic Law Predicting chemical/physical properties Electron Configuration Location of special groups on periodic table Chapter 5 Course: Technical Chemistry BENCHMARK SKILLS Classify elements according groups Apply rules for periodic trends (atomic radius, ionic radius, metallic character, electronegativity, electron affinity, ionization energy) Explain contributions of Moseley and Mendeleev in the development of the periodic table Explain how periodic law can be used to predict physical/chemical properties of elements Locate blocks of the periodic table ASSESSMENT Interactive Notetaking Quiz *Groups Flame Test Lab Ch 5 Review Test Colored periodic tables: Groups and SPDF blocks Foldable: Lewis dot structures Foldable: special groups Foldable: Blocks Foldable: Periodic Trends Atomic Radii, ionization energy, electron affinity, electronegativity Describe relationship between group configurations and group numbers Ch.5 Quizzes: Vocab quiz Describe locations of Special groups general properties of Lewis Dot alkali, alkaline earth, Blocks transition metals, Periodic Trends lanthanides, actinides, metalloids, chalogens, Ch.5 Review halogens, noble gases, WARREN HILLS REGIONAL SCHOOLS: COURSE OF STUDY/CURRICULUM MAP Teacher:DeVivo/Luff/Hodge CONTENT Chapter 6 Molecular Geometry Electron Dot Structure Types of Bonding Ionic/Polar Covalent/Nonpolar Covalent Course: Technical Chemistry BENCHMARK 5.1. 12A. 5.1. 12B. 5.3. 12B. 5.3. 12C. 5.6. 12A. 3 1 1 1,2,3,4,5,7,8 SKILLS elements existing as diatomic models ASSESSMENT Ch.5 Test Determine structure of selected molecules Molecular Geometry Lab Draw Lewis Dot Structures for elements and compounds Lewis Dot Structure Quiz Bonding Quiz Classify Bonds Chapter 6 Review Writing Formulas and Naming Ionic and Covalent Compounds Molar Mass % Composition Empirical/ Molecular Formula Chapter 7 5.1. 12A. 3,4 5.1. 12B. 1 5.2. 12C. 1 5.3. Classify compounds as being ionic or covalent Chapter 6 Test Quizzes *Vocabulary *Naming and Formulawriting for ionic compounds *Naming and Formulawriting for covalent compounds *Molar mass *% Composition *Empirical/Molecular Formulas Lab % water in a Hydrate Propose empirical and molecular formulas for compounds Tests *Formula Writing and Naming Determine the formula for a ionic and covalent compounds Determine the molar mass of given pure substances Analyze the percent composition of a compound WARREN HILLS REGIONAL SCHOOLS: COURSE OF STUDY/CURRICULUM MAP Teacher:DeVivo/Luff/Hodge CONTENT Reaction Types *Synthesis *Decomposition *Single Displacement *Double Displacement *Combustion Balancing Equations Course: Technical Chemistry BENCHMARK 5.4. 12B. 5.4. 12C. SKILLS Classify reactions Produce balanced chemical equations Develop a balanced chemical equation from a description of a chemical reaction Chapter 8 Identify the different types of reactions Predict the products of a reaction Stoichiometry Chapter 9 5.3. 12C. 5.6. 12A. 5.6. 12B. Perform a lab demonstrating all types of chemical reactions Perform stoichiometry calculations *mole/mole *mole/mass *mass/mole *mass/mass *limiting reactant *percent yield *percent composition ASSESSMENT *% composition, molar mass, empirical/ molecular formulas Quizzes Vocabulary Balancing Equations Reaction Types Worksheets Lab Types of Chemical Reactions *Report Test Quizzes *mole/mole *mole/mass *mass/mole *mass/mass Lab: Making copper(II)carbonate WARREN HILLS REGIONAL SCHOOLS: COURSE OF STUDY/CURRICULUM MAP Teacher:DeVivo/Luff/Hodge Course: Technical Chemistry CONTENT BENCHMARK SKILLS ASSESSMENT *mass/mass Chapter Review Test Pressure and Temperature Conversions Chapter 10 5.1. 5.1. 5.3. 5.6. 12A. 12C. 12B. 12A. Gas Laws *Boyle *Charles *Gay-Lussacs *Combined Gas *Dalton Perform pressure and temperature conversions Perform gas law calculations *Boyle *Charles *Gay-Lussacs *Combined Gas *Dalton Quizzes *Vocabulary *Conversions Pressure and temperature *Boyle *Charles *Gay-Lussacs *Combined Gas *Dalton Labs *Preparation of Gases *Gas Laws Chapter Review Test Ideal Gas Law Avogadro’s Law Chapter 11 Molecular Weight of a Gas 5.1. 5.1. 5.1. 5.6. 12A. 12B. 12C 12A. Perform Gas Law Calculations *Ideal Gas Law *Avogadro’s Law *Molecular Weight of a Gas *Density of a Gas Quizzes *Vocabulary *Molecular Weight of a Gas *Density of a gas **Ideal Gas Law *Avogadro’s Law Density of a Gas Lab *Molecular Weight of a WARREN HILLS REGIONAL SCHOOLS: COURSE OF STUDY/CURRICULUM MAP Teacher:DeVivo/Luff/Hodge Course: Technical Chemistry CONTENT BENCHMARK SKILLS ASSESSMENT Gas *% Oxygen in Air Test Phase Changes Chapter 12 Chapter 13 Solutions *saturated *unsaturated *supersaturated 5.1. 5.1. 5.1. 5.6. 12A. 12B. 12C. 12A. Determine the state of matter using a phase diagram Quest 5.1. 12A. 5.1. 12B. 5.1. 12C. Classify solutions as saturated, unsaturated, supersaturated Solubility Graph Perform molarity calculations Test Name acids and bases Classify solutions as being acidic, basic, or neutral Perform pH and pOH calculations Evaluate indicators for their uses Predict the products of neutralization reactions and balance the resulting equations Quizzes *Vocabulary *pH, pOH *Neutralization reactions *Naming acids and bases *Classification of Acids and Bases as weak or strong Molarity Acids/Bases Neutralization Reactions Indicators pH pOH Chapter 15/16 5.3. 12C. 5.6. 12A. Lab *Energy and Phase Change Chapter Review Lab *Indicators *Titration Chapter Review WARREN HILLS REGIONAL SCHOOLS: COURSE OF STUDY/CURRICULUM MAP Teacher:DeVivo/Luff/Hodge CONTENT Course: Technical Chemistry BENCHMARK SKILLS ASSESSMENT Test Naming and drawing structures of organic compounds Chapter 20/21 5.1. 12A. 5.6. 12A. Evaluate structures for functional groups Propose names for organic structures Produce structures for organic names Draw and name isomers for selected organic compounds Quizzes *Vocabulary *Identify functional groups alkane, alkene, alkyne, alcohol, aldehyde, ketone, carboxylic acid, ester, ether *Naming organic structures alkanes, alkenes, alkynes, alcohols *Draw organic structures alkanes, alkenes, alkynes, alcohols *Isomers Chapter Review Lab Modeling Test