WEDNESDAY, DECEMBER 19, 2001
advertisement
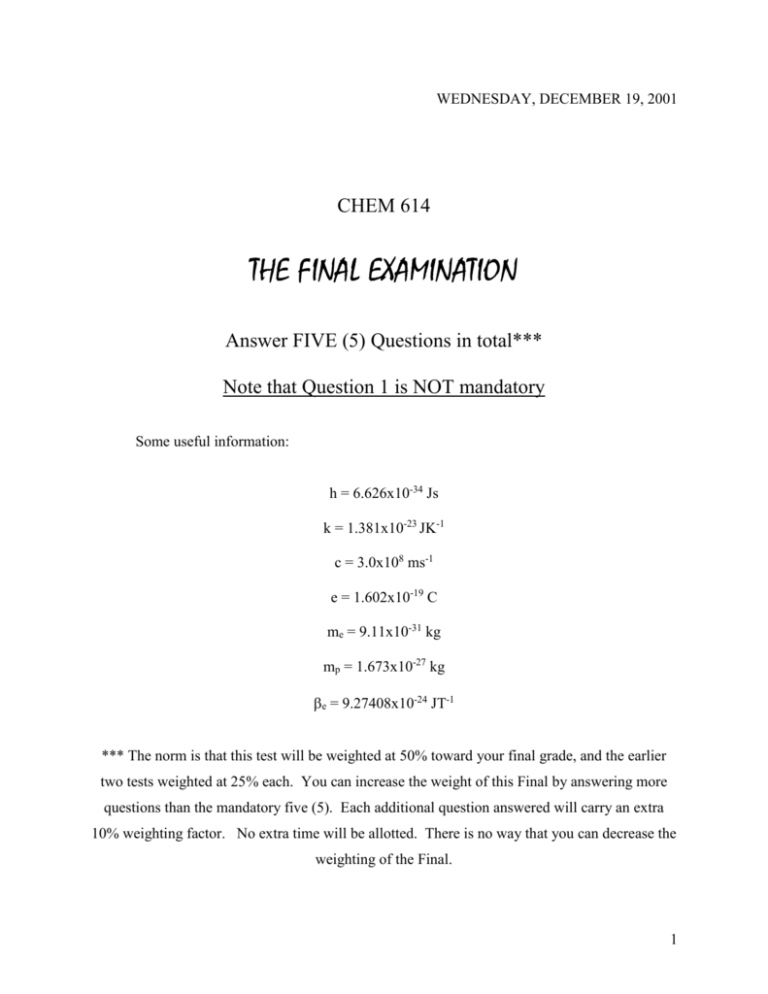
WEDNESDAY, DECEMBER 19, 2001 CHEM 614 THE FINAL EXAMINATION Answer FIVE (5) Questions in total*** Note that Question 1 is NOT mandatory Some useful information: h = 6.626x10-34 Js k = 1.381x10-23 JK-1 c = 3.0x108 ms-1 e = 1.602x10-19 C me = 9.11x10-31 kg mp = 1.673x10-27 kg e = 9.27408x10-24 JT-1 *** The norm is that this test will be weighted at 50% toward your final grade, and the earlier two tests weighted at 25% each. You can increase the weight of this Final by answering more questions than the mandatory five (5). Each additional question answered will carry an extra 10% weighting factor. No extra time will be allotted. There is no way that you can decrease the weighting of the Final. 1 1: Answer TRUE or FALSE to the following: T/F The postulates of quantum mechanics have been proven to be true. _____ Bonding -MOs in O2 are gerade. _____ Light at 600 nm will generate photoelectrons from a metal of work function 2.6 ev. _____ H1s H 2 p 0 _____ The Hückel MO method assumes -separability. _____ The Hartree-Fock energy is always a very close approximation to the true value. _____ The 3D multiplet is composed of 12 states. _____ For a particle in a cubical enclosure the states 1, 2,3 , 1,3, 2 ,and 3, 2,1 are degenerate. _____ If ˆ d nHˆ * m * d , then it is always true that m * Hn ˆ nHˆ * m * m * Hn _____ In the 1s2s electronic configuration the lowest energy level is singly degenerate. _____ All angular momentum operators commute. _____ The eigenfunctions of the position operator are Dirac delta functions. _____ A magnetic field of 2 T splits the 2p0 and 2p+1 levels in the H atom by ca 10-19 J. _____ f ( r ) e ar is an eigenfunction of the derivative operator. 2 Terms having the greater spin multiplicity are lower in energy. _____ _____ ♣♦♥♠♣♦♥♠♣♦♥♠♣♦♥♠♣♦♥♠ 2 2. Write clear explanatory paragraphs on five of the following subjects: a) The Born-Oppenheimer Approximation b) Radial Distribution Functions c) The zero point energy of a harmonic oscillator. d) Slater-type orbitals. e) Inversion symmetry in diatomic molecules. f) The Exclusion Principle. g) The Photoelectric Effect. h) Hermiticity. i) Configuration Interaction. j) The Zeeman effect in many-electron atoms. k) The NH3 potential energy curve. l) The Uncertainty Principle. m) Orthonormality. n) The independent electron approximation. o) The Hartree-Fock limit. p) Bonding in BeH2. ♣♦♥♠♣♦♥♠♣♦♥♠♣♦♥♠♣♦♥♠ 3 Write clear explanatory paragraphs about another 2. five of the subjects listed in Question ♣♦♥♠♣♦♥♠♣♦♥♠♣♦♥♠♣♦♥♠ 3 4 (a) Explain the significant approximations employed in the Hückel MO (HMO) method for treating electrons in unsaturated hydrocarbons. (b) The Hückel determinantal equation for the allyl radical can be written as x 1 0 1 x 1 0 0 1 x Explain the element x in this determinant. (c) Explain the terms coulomb integral, exchange (resonance) integral and overlap integral as they apply to the HMO method. (d) Use a diagrammatic procedure to find the HMO energy levels for benzene. (e) What is E for benzene? (f) What acyclic compound can be represented using an identical diagram that you used for benzene? (g) What is E for this second compound? ♣♦♥♠♣♦♥♠♣♦♥♠♣♦♥♠♣♦♥♠ 5 (a) Briefly outline the basics of the variational procedure for calculating the approximate energy of a molecule. (b) Write down the hamiltonian operator that you would employ to obtain the electronic energy of H2, explaining any approximations/assumptions you need to use. (c) What is the electronic configuration of the lowest energy state of the hydrogen molecule? (d) Use a minimal basis set of orthonormal hydrogen atom AOs to construct the Slater determinant for H2. (e) Calculate the normalization constant for the molecular orbital in (d). (Hint: the answer will be the square of the normalization constant for the H2+ MO). (f) Construct the secular determinant for the MO in (d) and generate an equation for evaluating the electronic energy of H2. (g) The PE curve for the lowest energy state of H2 has a minimum at 1.4 au. If the calculated electronic energy at that distance is –1.804 au, evaluate the corresponding predicted bond dissociation energy. Is this higher or lower than the experimental value of 0.1746 au? ♣♦♥♠♣♦♥♠♣♦♥♠♣♦♥♠♣♦♥♠ 4 6 (a) Find the condition that a linear combination of eigenfunctions of Ĥ is also an eigenfunction of Ĥ . (b) Explain clearly whether the 1s state or the 2s state of the hydrogen atom will be more polarizable in a uniform electric field. (c) A variation theory calculation for the hydrogen atom is started with the normalized trial function (in au) (2 )7 / 4 6! r 2 e r At some point during the process the calculation generates the following closed bracket Hˆ 2 /10 / 3 Use this information to find the minimum value for and the corresponding orbital energy (in au). (d) Write down the exact form of Ĥ that you would need to use in the preceding equation. ♣♦♥♠♣♦♥♠♣♦♥♠♣♦♥♠♣♦♥♠ 7 (a) Find the conditions under which the general ket q can be simultaneously an eigenket of the operators  and B̂ . (b) Consider the case of a particle in a one-dimensional box ( V at the walls). A nonstationary state of the system may be defined by a linear combination of time-dependent functions of the type ( q) f (t ) where q and t represent spatial and temporal coordinates, respectively. Construct a wavefunction ( q, t ) , which is a normalized 50-50 mixture of two of the above functions, and so obtain an expression for 2 as a function of q, t, and the difference in the energy eigenvalues corresponding to the two functions. ♣♦♥♠♣♦♥♠♣♦♥♠♣♦♥♠♣♦♥♠ 5 8 (a) Write down the Schrödinger equation for a one-dimensional simple harmonic oscillator. (b) Normalized eigenfunctions of the hamiltonian for this system are found to be 1/ 2 1 n ( y) n 2 n! H n ( y ) exp( y 2 / 2) where y x and n = 0, 1, 2, ... The above expression contains three significant parts. What are these and what role do they play in determining the shape of the wavefunction? (c) Make careful sketches of the functions having n = 0 and n = 2. (d) The n = 1 to n = 2 transition in 1H127I occurs at 2308 cm-1. Calculate the force constant of the bond. (e) Would you expect the same transition in 2H127I to be at higher or lower wave numbers? ♣♦♥♠♣♦♥♠♣♦♥♠♣♦♥♠♣♦♥♠ 9. For the case of a particle moving in a ring two wavefunctions are under consideration (2 ) 1/ 2 exp(i 2 ) and ( )1/ 2 cos(3 ) (a) Are both these functions acceptable? Explain your answer. (b) Write down the hamiltonian for the motion. (c) For the acceptable function write down the Schrödinger equation and evaluate the eigenvalue (in molecular units). (d) Determine whether the acceptable function is an eigenfunction of the angular momentum operator. ♣♦♥♠♣♦♥♠♣♦♥♠♣♦♥♠♣♦♥♠ 6 7