Electron Configuration Review
advertisement

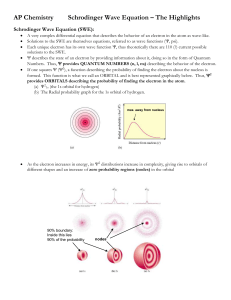
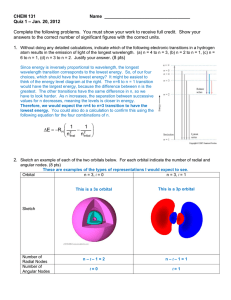
Electron Configuration Review 1. Write the electron configuration (the long way) for the following elements: a. Silicon (Si) b. Palladium (Pd) c. Selenium (Se) 2. Write the electron configuration (the short way) for the following elements: a. Barium (Ba) b. Indium (In) c. Fe+3 3. Which element has the following electron configuration? a. 1s22s22p63s23p64s23d2 b. 1s22s22p63s23p64s23d104p1 c. 1s22s22p63s23p64s23d104p65s2 4. Which of the following electron configurations shows an atom in the excited stated (Which one is not following Aufbau’s Principle)? a. 1s22s22p63s23p5 b. 1s22s22p63s1 c. 1s22s22p63s23p64s25s2 5. Calculate the wavelength of light which gives off 2.87x10-19 J of energy (Show all work) 6. What is the energy (E) of…(show all work) a. a photon of blue light that has a wavelength (λ) of 500 nm? b. a photon of violet light that has a frequency (ν)of 7.5x1014 s-1? 7. Draw the orbital diagram for (refer to page 367) a. Chlorine b. Titanium 8. What type of sublevels (s, p, d, f) does each energy level contain? 9. How many orbitals does each sublevel (s, p, d, f) contain? 10. What is the shape of the… a. s orbital b. p orbital c. d orbital 11. Explain how light is emitted from electrons in an atom?





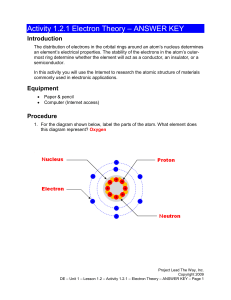
![6) cobalt [Ar] 4s 2 3d 7](http://s2.studylib.net/store/data/009918562_1-1950b3428f2f6bf78209e86f923b4abf-300x300.png)