VSEPR worksheet - mvhs
advertisement
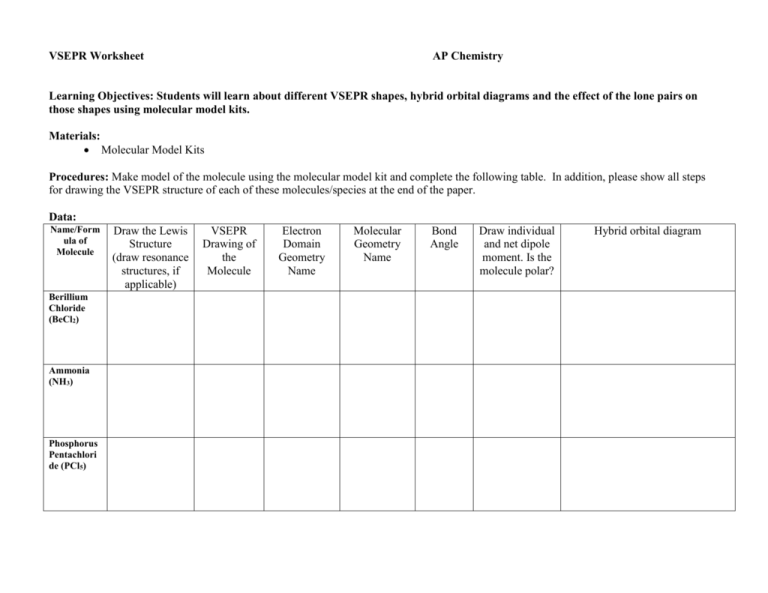
VSEPR Worksheet AP Chemistry Learning Objectives: Students will learn about different VSEPR shapes, hybrid orbital diagrams and the effect of the lone pairs on those shapes using molecular model kits. Materials: Molecular Model Kits Procedures: Make model of the molecule using the molecular model kit and complete the following table. In addition, please show all steps for drawing the VSEPR structure of each of these molecules/species at the end of the paper. Data: Name/Form ula of Molecule Berillium Chloride (BeCl2) Ammonia (NH3) Phosphorus Pentachlori de (PCl5) Draw the Lewis Structure (draw resonance structures, if applicable) VSEPR Drawing of the Molecule Electron Domain Geometry Name Molecular Geometry Name Bond Angle Draw individual and net dipole moment. Is the molecule polar? Hybrid orbital diagram Name/Form ula of Molecule Draw the Lewis Structure (draw resonance structures, if applicable) VSEPR Drawing of the Molecule Electron Domain Geometry Name Molecular Geometry Name Bond Angle Draw individual and net dipole moment. Is the molecule polar? Carbonate ion (CO32-) Sulfer Trioxide (SO3) Tetrachloro idodide ion (ICl4-) Discussion Questions: 1. How do the bond angles differ on a molecule with non-bonding electron pairs around the central atom? 2. Name two elements that can be central atoms with less than a full octet. 3. Predict the bond angles around the central atom in the molecule, ClF4+ ion. 4. Why does the molecule, BF3, contain only single bonds giving boron an incomplete octet? Hybrid orbital diagram







