Density Notes and Homework Package 2013-KEY
advertisement

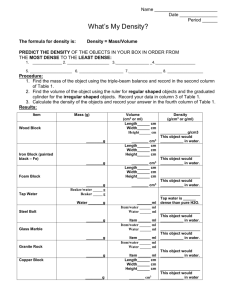
NAME__________________________ Section_______ Date ______________ Mass, Volume and Density: On-line tutorial and practice Instructions: 1. Type the following url in the address bar of your web browser http://ippex.pppl.gov/interactive/ 2. Fill in the blanks/ answer the questions as you go through the web site. 1. Mass, Volume and Density are physical properties of an object. 2. The mass of an object is its amount of matter. 3. Volume is the amount of space an object occupies. 4. The volume of regular solid can be used using mathematical formulas that depend on the specific geometry of the object. 5. The formula to measure the volume of a box is: length X width X height. 6. You can find the volume of an irregular shaped item like a rock can be measured through fluid displacement using a graduated cylinder and water. 7. The volume of the rock is 20 ml or 20 cm3 8. Density is a physical property of matter. Density is defined as: The amount of mass per unit of volume 9. The unit of density is: g/cm3 10. The bowling ball is the more dense ball because even though they both have the same volume, the bowling ball has more mass. 11. Write the formula for calculating the density of an object. Mass/volume 12. What is the density of the wood block? (show your work by writing the formula) 0.9g/cm3 This page is worth 13 points Page 1 of 4 13.The density of water is 1g/mL. What do you notice about the density of the objects that will float in water? The objects that float in water are less dense than water. 14. Most substances become more dense when they freeze. Water is one of the few substances that become less dense when it freezes. Why is this important for life on earth? (Hint: Where does ice form when lakes freeze over?) There are many possible answers. One: If lakes/rivers/seas froze from the bottom up, all the life on the bottom of these bodies would freeze and die. 15. You are given a box as large as a refrigerator full of feathers and one small marble. Which is more dense, the box of feathers or the marble? Explain. The marble has a greater density. 100 tons of feathers would still be less dense than a small glass marble, because glass has a greater density than feathers. Neither the mass of the feathers nor the volume of the feathers is what matters. It is the proportion that matters. A sample of feathers of the same volume as the marble will have less mass than the marble. 16. What is the density of an object that has a mass of 926g and a length of 6cm, a width of 4 cm and a height of 2 cm? (VOLUME = length X width X height) What object is it? (show your work) Volume = 6 X 4 X 2 = 48 cm3 Mass = 926g Density = 19g/cm3 The object is gold This page is worth 9 points total Page 2 of 4 NAME__________________________ Section_______ Date ______________ Virtual Density Lab Virtual Density Lab Data This table is worth 15 points Object Mass (g) Volume (mL) Density (g/ml) Float in water? Float in Oil? Float in SaltWater? Charcoal 3.12 15 0.208 yes yes yes Egg 9.18 9 1.02 no no yes Gold 250.9 13 19.03 no no no Iron 39.0 5 7.8 no no no Oak wood 11.16 12 0.93 yes no yes Discussion Questions These three questions are worth 4 points total 1. What is the density of water? 1g/mL 2. List each object, with its density, in order from the object that is most dense to the object that is least dense. 1. gold 2. iron 3. egg 4. charcoal 5. wood 3. What do you notice about the density of objects that float in water and the density of objects that sink in water? The objects that float are less dense, and the objects that sink are more dense. Page 3 of 4 4. What can you assume about the density of salt-water compared to the density of water? Explain. Salt water is more dense than is plain water, because although the egg sinks in plain water, it floats in salt water. The salt water must be more dense than the egg if it is able to support it. 5. Complete the following sentence: If an object’s density is greater than 1 g/ml than the object’s mass will be greater than (less than, greater than) the volume and if an object’s density is less than 1 g/mL than the object’s mass will be less than (less than, greater than) the volume. 6. If you had 100mL of water, what would the mass of the water be? The water would have a mass of 100g. These questions are worth 4 points total Page 4 of 4