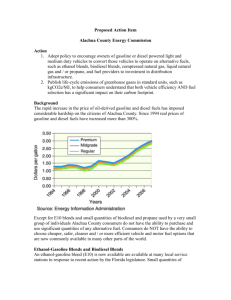
The properties of gasoline kerosene and diesel
advertisement
The properties of petrol (gasoline), kerosene and diesel What you already know: How these fuels are derived from mineral oil and the differences in their molecular structure and boiling points. Just to check, fill in the missing data in the following table: Kind of fuel Gasoline Kerosene Diesel Light fuel oil 1 Boiling range What you are now going to find out: How the different molecular structures of various forms of petrol, of kerosene and of diesel affect their other properties. 1) 2) 3) 4) Size of molecules1 Number of Carbon-atoms Next, give some constitution formulae (at least 3 per fuel) of compounds that are contained in these fuels. (Best use the type consisting only of C-C-bonds). What is the difference between open-chain and cyclic saturated hydrocarbons? – What are isomers? Work sheet: Mineral oil based fuels Now make sure that you know, how the three types of engines (petrol-motor, jet engine, diesel-motor) work! You might consult the following link: http://auto.howstuffworks.com/engine.htm Melting ranges of gasoline and diesel Flammability of gasoline and diesel Difference between high and low octane gasoline Aromatic hydrocarbons in gasoline Ad 1) If your family owns a car with a diesel-engine, you might have encountered the situation that the car did not work on account of the cold on a day with temperatures far below zero. What you need: Petrol and light fuel oil (just a few millilitres), test tubes, a thermometer, ice, salt and a basin Put crushed ice into the basin and prepare a freezing mixture by adding copious amounts of salt. The temperature should fall to approximately -20°. Then stick two test tubes – one filled with gasoline the other with diesel – into it. – What do you observe? …………………………………………………………………………………………………. E. Langer 2006 1/3 What is the reason for taking light fuel oil instead of diesel? – Diesel contains additives that are harmful, so that you should avoid inhaling it or letting it get into contact with your skin! (Keep this in mind, when refuelling your(?) car at the service station!) Ad 2) You ought to know that petrol must not ignite by itself in the petrol-engine, while diesel in the diesel-motor has to do so to guarantee its function. Therefore one might assume that diesel can be ignited more easily than petrol. Let’s check! You need: Petrol and light fuel oil or liquid paraffin, two porcelain pots and matches (preferably long ones!) – but there is no need to use a burner. Safety goggles and protection gloves! surface of the liquids. What do you observe? Fill a few millilitres of the two fuels into separate pots, put them apart by some thirty centimetres and hold a burning match above the …………………………………………………………………………………………………. Please explain! ………………………………………………………………………………………………… ………………………………………………………………………………………………… ………………………………………………………………………………………………… Work sheet: Mineral oil based fuels Ad 3) Please recapitulate what you’ve learnt about the reforming process! Why is self-ignition less probable with branched and cyclic hydrocarbons? How is the octane number defined? – Which are the two reference compounds? What you need: Two porcelain pots, heptane, 2,2,4-threemethyloctane (What’s its trivial name?), matches; Safety goggles and protection gloves! Fill a few millilitres of the two fuels into separate pots, put them apart by some thirty centimetres and ignite them by means of a match! Now watch the two components of petrol while burning! – What do you observe? ………………………………………………………………………………………………. Please explain! ……………………………………………………………………………………………… ……………………………………………………………………………………………… ……………………………………………………………………………………………… Ad 4) First of all you’ll have to find out (or repeat) what aromatic hydrocarbons are. A brief introduction may be found at the following link: http://library.thinkquest.org/3659/orgchem/aromatic.html Fuels contain about 2 % of aromatic compounds. This improves their anti-knock-qualities, but many aromatic hydrocarbons (particularly benzene itself and certain condensed aromatics) are carcinogenic. E. Langer 2006 2/3 You need: different types of gasoline, toluene, aluminium chloride (AlCl3 - corrosive!), chloroform and some test tubes. Please work in a fume hood with working ventilation! Work with goggles and gloves! First of all put a small portion of AlCl3 into a test tube and heat it with the burner, so that it sublimates and forms a deposit on the wall of the tube. Now dilute a few droplets of toluene with 2-3 millilitres of chloroform and add a little bit of this solution to the AlCl3 deposit by means of a pipette. – What do you observe? derive constitution formula petrol (brit.) flammability copious refuel harmful ignite recapitulate fume hood dilute Gasoline (amer.) abundant noxious repeat Abzug, chemischer Herd Please write down additional new words at the backside of the pages! Work in pairs and be aware of the necessary safety precautions!!! ………………………………………………………………………………………………. Explanation: Aromatic compounds form intensely coloured adducts with Lewis acids like AlCl3. Now repeat the whole sequence but use gasoline instead of the toluene solution. Observation: ……………………………………………………………………………………………... (You can try other aromatic compounds like naphthalene as well!) Experiments and pictures have been taken from the website: http://dc2.uni-bielefeld.de/dc2/auto/index.html Work sheet: Mineral oil based fuels E. Langer 2006 3/3