Heat Capacity Practice
advertisement
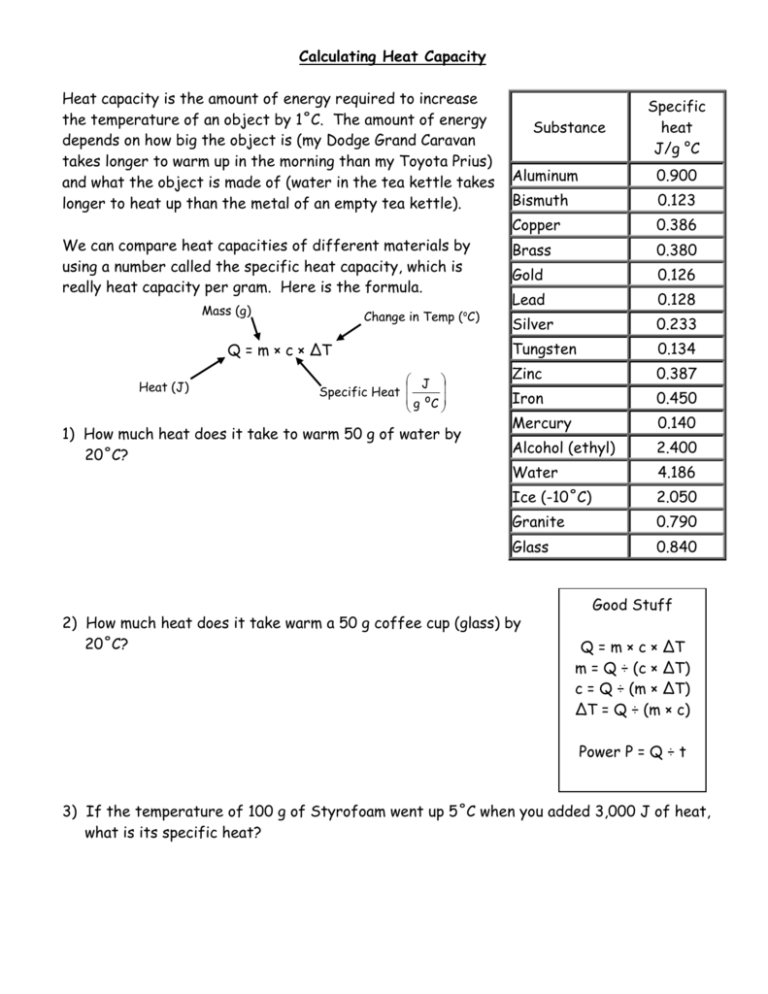
Calculating Heat Capacity Heat capacity is the amount of energy required to increase the temperature of an object by 1˚C. The amount of energy depends on how big the object is (my Dodge Grand Caravan takes longer to warm up in the morning than my Toyota Prius) and what the object is made of (water in the tea kettle takes longer to heat up than the metal of an empty tea kettle). We can compare heat capacities of different materials by using a number called the specific heat capacity, which is really heat capacity per gram. Here is the formula. Mass (g) o Change in Temp ( C) Q = m × c × ΔT Heat (J) J Specific Heat g oC 1) How much heat does it take to warm 50 g of water by 20˚C? Substance Specific heat J/g °C Aluminum 0.900 Bismuth 0.123 Copper 0.386 Brass 0.380 Gold 0.126 Lead 0.128 Silver 0.233 Tungsten 0.134 Zinc 0.387 Iron 0.450 Mercury 0.140 Alcohol (ethyl) 2.400 Water 4.186 Ice (-10˚C) 2.050 Granite 0.790 Glass 0.840 2) How much heat does it take warm a 50 g coffee cup (glass) by 20˚C? Good Stuff Q = m × c × ΔT m = Q ÷ (c × ΔT) c = Q ÷ (m × ΔT) ΔT = Q ÷ (m × c) Power P = Q ÷ t 3) If the temperature of 100 g of Styrofoam went up 5˚C when you added 3,000 J of heat, what is its specific heat? 4) The sun shines on a 1000 g rock (granite) and adds 1500 J of heat to the rock. How much does its temperature rise? 5) Making Tea A. A kettle (400 g of iron) is used to boil 1 liter of water (1000 g). If the kettle and the water both start at 20˚C, how much heat does the stove have to produce for the water to come to a boil (assuming all of the heat goes to warming the kettle and the water)? B. If you wanted to make your tea in 1 minute (60 s), how much power does the burner need to generate?