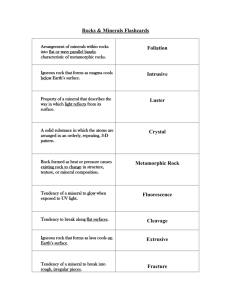
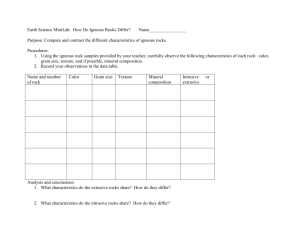
EESUnit 2 With LEP (6-27-08)
advertisement

COURSE: Earth/Environmental Science I. Grade Level/Unit Number: 9 - 12 Unit 3 II: Unit Title: III. Unit Length: 15 days (on a 90 min per day block schedule) IV. Major Learning Outcomes: LITHOSPHERE - Part 1 – Minerals - Part 2 – Rocks - Part 3 – Plate Tectonics - Part 4 – Earthquakes - Part 5 – Economic Development of the Earth The student will gain an understanding of: The role of inquiry in the investigation and classification of minerals The importance of safety when conducting scientific investigation The forces that drive the rock cycle and are responsible for mineral formations The historical development of the theory of plate tectonics. The interdependence between human and natural systems. Earth's internal structure. The importance and impact of the economic development of earth's finite rock, mineral, soil, fossil fuel and other natural resources to society and our daily lives V. Content Objectives Included (with RBT Tags): Objective Number 1.01 1.02 1.03 Objective Identify questions and problems in the earth and environmental sciences that can be answered through scientific investigations Design and conduct scientific investigations to answer questions related to earth and environmental science. Create testable hypotheses Identify variables. Use a control or comparison group when appropriate. Select and use appropriate measurement tools. Collect and record data. Organize data into charts and graphs. Analyze and interpret data. Communicate findings. Evaluate the uses of satellite images and imaging techniques in the earth and environmental sciences. Earth/Environmental Science- Unit 3 DRAFT RBT Tag B1 B6 A5 1 1.04 1.05 1.06 Apply safety procedures in the laboratory and in field studies: C3 Recognize and avoid potential hazards. Safely manipulate materials and equipment needed for scientific investigations. Analyze reports of scientific investigations and environmental issues C4 from an informed scientifically literate viewpoint including considerations of: Appropriate sample. Adequacy of experimental controls. Replication of findings. Alternative interpretations of the data. C5 Identify and evaluate a range of possible solutions to earth and environmental issues at the local, national, and global level including considerations of: 2.01 2.02 2.03 2.04 2.05 2.06 Interdependent human and natural systems. Diverse perspectives. Short and long range impacts. Economic development, environmental quality and sustainability. Opportunities for and consequences of personal decisions. Risks and benefits of technological advances. Analyze the dependence of the physical properties of minerals on the arrangement and bonding of their atoms. Analyze the historical development of the theory of plate tectonics. B4 B4 Investigate and analyze the processes responsible for the rock cycle: Analyze the origin, texture and mineral composition of rocks. Trace the path of elements through the rock cycle. Relate rock formation to plate tectonics. Identify forms of energy that drive the rock cycle. Analyze the relationship between the rock cycle and processes in the atmosphere and hydrosphere. Analyze seismic waves including velocity and refraction to: Infer Earth's internal structure. Locate earthquake epicenters. Measure earthquake magnitude. Evaluate the level of seismic activity in North Carolina. B4 Create and interpret topographic, soil and geologic maps using scale and legends. Investigate and analyze the importance and impact of the economic development of earth's finite rock, mineral, soil, fossil fuel and other natural resources to society and our daily lives: Availability. Geographic distribution. Conservation/Stewardship. Recycling. B6 Earth/Environmental Science- Unit 3 DRAFT C4 B5 2 Environmental impact. Challenge of rehabilitation of disturbed lands. Analyze the sources and impacts of society's use of energy. Renewable and non-renewable sources. The impact of human choices on Earth and its systems. 2.07 B5 VI. English Language Development Objectives (ELD) Included: NC English Language Proficiency (ELP) Standard 4 (2008) for Limited English Proficiency Students (LEP)- English Language learners communicate information, ideas, and concepts necessary for academic success in the content area of science. Suggestions for modified instruction and scaffolding for LEP students and/or students who need additional support are embedded in the unit plan and/or are added at the end of the corresponding section of the lessons. The amount of scaffolding needed will depend on the level of English proficiency of each LEP student. Therefore, novice level students will need more support with the language needed to understand and demonstrate the acquisition of concepts than intermediate or advanced students. VII. Materials/Equipment Needed: Activity Materials PART 1 - MINERALS Introductory Activity: Salt Sand and Sand in Water Salt Spoon Cups Clear bowl (if done as demo) Basic Mineral Identification: Parts 1-4 Sets of minerals to include: talc, gypsum, calcite, fluorite, apatite, feldspar, quartz, topaz and *corundum Rock and mineral guidebooks Copper pennies Glass plates Hand lenses Streak plate Basic Mineral Identification: Part 5 Clear rhombohedral calcite specimen Clear ulexite specimen Thin pieces of muscovite Polarizing filters Scotch tape (the old-fashioned clear kind) Basic Mineral Identification: Part 6 100 mL graduated cylinder Scale Samples of galena, pyrite and copper Earth/Environmental Science- Unit 3 DRAFT 3 Rock and mineral guidebooks Mineral Families and Formation Mineral specimens Hand lenses Rock and mineral guidebooks AD Campaign for a Mineral poster board or construction paper markers rulers rock and mineral guidebooks names of individual minerals on note cards or paper Crystal Systems Prentice Hall Earth Science Laboratory Manuel PART 2 - ROCKS Rock Roulette 11 six-sided die Station signs Rock Identification Using Copies ROCK KEY from the web site Hand lenses Flow Charts Diluted hydrochloric acid Flow chart station questions printed on cards (one per station) The Melting Point of Computers with Internet access Rocks Igneous Rock Identification Computers with Internet access History Rocks Paper Pencil PART 3 – PLATE TECTONICS Oreo Cookie Activity Oreo cookies Napkins Locating Patterns of Earthquake and Volcano Distribution A world map per student showing latitude and longitude (on back) Four different colored pencils Pushing and Pulling Mountains Foam of different thickness and color (craft and camping pad foam) String Rope 4x4 piece of wood 2 feet long Earth/Environmental Science- Unit 3 DRAFT 4 Saw Scissors Evidence for Plate Tectonics Computers with Internet access Shoebox Glue Scissors Markers Patterns Edible Geology Three flavors of gelatin - maybe raspberry, lime, and lemon Boiling water Graham cracker Whipped cream Banana or canned fruit cocktail, drained Clear Pyrex or glass pan – (8 x 12 x 2) Measuring cup Spatula Refrigeration Slice of Earth PART 4 - EARTHQUAKES 2- 11 x 17 inch sheets of paper Meter stick ~ 70 cm long string with loop at one end Tape Pencil Finding the Epicenter of an Earthquake Drawing compass with pencil Graph that plots distance to epicenter (Xaxis) and difference in arrival time between P and S waves United States map Investigating the Speed of Earthquake Waves Four different colored pencils Graph of Seismic Wave Travel Time vs. Distance Earth/Environmental Science- Unit 3 DRAFT 5 Density of Igneous Rocks Modeling Liquification Design and Build a Simple Seismograph Hand lens Intrusive igneous rocks Tables from website Prentice Hall Earth Science Lab Manuel pp. 69-72 Prentice Hall Earth Science Lab Manuel pp. 73-78 Computers with Internet access Virtual Earthquake Computers with Internet access Locating the 1989 “World Series Earthquake” Epicenter PART 5 – ECONOMIC DEVELOPMENT OF EARTH Several varieties of chocolate chip cookies Chocolate Chip Cookie (high, medium and low-end) Mining Paper (“play”) money (10s, 5s, and 1s) Grid paper Toothpicks (flat and round) Paper clips Recovering Oil PRENTICE HALL EARTH SCIENCE LAB MANUEL (Chapter 4, Investigation 4A) Investigating Ore Deposits McDOUGALL-LITTELL EARTH SCIENCE LAB MANUEL (Chapter 7) Pizza box Magnetic & electronic stud finder Duct tape Assortment of magnetic and non-magnetic objects Graph paper USING DISASTER TO TEACH SCIENCE Computers Newspaper Article about Pipeline Fire Equation Balancing Tutor 3" x 5" card VIII. Detailed Content Description: Please see the detailed content description for each objective in the Earth and Environmental Support Document. The link to this downloadable document is in the Earth and Environmental Science Standard Course of Study at: http://www.ncpublicschools.org/curriculum/science/scos/2004/25earth Earth/Environmental Science- Unit 3 DRAFT 6 IX. Unit Notes Overview Of Unit Three This unit is focused on the formation and processes that govern Earth’s materials such as rocks, minerals, soil, mountain formation, and plate movement. Included are the impacts of human conditions on the availability of resources with their economical implications as well as the impact of plate movements. Specifically, students will gain an understanding of: basic mineral formation and human impact on the land due to mining the rock cycle and its effects on soils classification techniques earth’s physical structure and continued development the historical theory of plate tectonics plate movement and their impacts on the formation of mountains and basins historical relevance concerning North Carolina’s earthquake events In each unit, Goal 1 objectives which relate to the process of scientific investigation are included. In each of the units, students will be practicing the processes of science: observing, hypothesizing, collecting data, analyzing, and concluding. The unit guide gives an overview of the activities that are suggested to meet the Standard Course of Study Goals for Unit Three. The guide includes activities, teacher notes on how to weave the activities into the content, and supplementary notes related to other issues such as preparation time and time to complete the activity. If a teacher follows this unit s/he will have addressed the goals and objectives of the SCOS. However, teachers may want to substitute other activities that teach the same concept. Teachers should also refer to the support document for Earth/Environmental Science at http://www.ncpublicschools.org/curriculum/science/scos/2004/25earth for the detailed content description for each objective to be sure they are emphasizing the specified concepts for each objective. Essential Questions for Unit Three : The following are the essential questions for part 1 of this unit. Essential questions are those questions that lead to enduring understanding. These are the questions that students should be able to answer at some level years after the course. These questions are designed to incorporate multiple concepts. Students will work on answering these questions throughout the unit. Teachers are advised to put these questions up in a prominent place in the classroom and refer to them during the teaching of the unit. Part 1 - Minerals: 1) How do scientists conduct experiments safely? 2) How does the arrangement of atoms and bonding determine mineral properties? 3) What is the relationship between rocks and minerals? 4) What are the major types of chemical bonding? Earth/Environmental Science- Unit 3 DRAFT 7 5) How are minerals classified? 6) How do geologic conditions affect mineral formation? Part 2 – Rocks 1) What are the processes and products of the rock cycle? 2) How does analysis of texture and composition of igneous rocks infer the rock’s origin? 3) Describe the forms of energy that drive the rock cycle. 4) How do processes in the atmosphere and hydrosphere affect the rock cycle? Part 3 – Plate tectonics 1) What are the seismic characteristics of the different types of plate boundaries? 2) How do volcanoes form at plate boundaries? 3) What are the rock types at plate boundaries? 4) How are minerals evidence for past and present plate tectonic activity? Part 4 – Earthquakes 1) What factors determine faults or folding of the crust? 2) How does the density of a material affect the speed and intensity of a seismic wave? 3) How are seismic waves analyzed to determine an earthquake’s epicenter and intensity? 4) Explain the connection between plate boundaries and earthquakes. 5) Describe the level of seismic activity in North Carolina. 6) What are the distinguishing features of crust, mantle, outer and inner core? Part 5 – Economic Development of the Earth 1) Where are minerals found? 2) How are minerals mined? 3) What impact does mining have on the surface of the Earth? 4) Does the cost of extraction justify the means? 5) How are minerals used in my everyday life? What impact does this have on the resource? 6) How do “I” impact the environment? My neighborhood? My daily path to school? Modified Activities for LEP Students: Those activities marked with a have a modified version or notes designed to assist teachers in supporting students who are English language learners. Teachers should also consult the Department of Public Instruction website for English as a Second Language at: http://www.ncpublicschools.org/curriculum/esl/ to find additional resources. Earth/Environmental Science- Unit 3 DRAFT 8 LEP Accommodation Considerations The following are general suggestions for accommodating English second language: 1. Assess the prior knowledge of your LEP student and make sure that he or she has adequate background information in order to execute this activity. 2. Provide graphic organizers or roadmaps illustrating the specific procedures and expectations of each activity. 3. Provide highlighted text which target key vocabulary and concepts. Review this text prior to activity. 4. Elicit verbal response of understanding from student. For, example, “Explain to (or show me) me what you need to do next.” 5. Include marginal notes in activity outline to re-emphasize terms and concepts. 6. Provide visual demonstration in conjunction with verbal instructions 7. Provide immediate feedback and or assessment in order to reinforce objectives. 8. Provide for alternate forms of assessment such as concept maps, graphic organizers, verbal explanations, written explanations, or actual performance rather than strictly pen and paper tests. 9. Provide LEP students the opportunity to peer tutor, pairing those who are on different proficiency levels. 10. Provide opportunities to demonstrate effective test- taking strategies, regularly exposing students to sample questions. Computer Based Activities: Some of the recommended activities are computer based and require students to visit various internet sites and view animations of various processes and identification guides. These animations require various players and plug-ins which may or may not already be installed on your computers. Additionally some districts have firewalls that block downloading these types of files. Before assigning these activities to students it is essential for the teacher to try them on the computers that the students will use and to consult with the technology or media specialist if there are issues. Some of these animations also have sound. Teachers may wish to provide headphones if possible. X. Global Content: Aligned with 21st Century Skills One of the goals of the unit plans is to provide strategies that will enable educators to develop the 21st Century skills for their students. As much as students need to master the NCSOS goals and objectives, they need to master the skills that develop problem solving strategies, as well as the creativity and innovative thinking skills that have become critical in today’s increasingly interconnected workforce and society. The Earth/Environmental Science- Unit 3 DRAFT 9 Partnership for 21st Century Skills website is provided below for more information about the skills and resources related to the 21st Century classroom. http://www.21stcenturyskills.org/index.php?option=com_content&task=view&id=27&Ite mid=120 NC SCS Biology 1.01, 1.02, 2.01, 2.02, 2.04 21st Century Skills Communication Skills Conveying thought or opinions effectively When presenting information, distinguishing between relevant and irrelevant information Explaining a concept to others Interviewing others or being interviewed Computer Knowledge Using word-processing and database programs Developing visual aides for presentations Using a computer for communication Learning new software programs Employability Skills Assuming responsibility for own learning Persisting until job is completed Working independently Developing career interest/goals Responding to criticism or questions Information-retrieval Skills Searching for information via the computer Searching for print information Searching for information using community members Language Skills - Reading Following written directions Earth/Environmental Science- Unit 3 DRAFT Activity Most of the activities can be presented as opportunities for students to follow written directions. The teacher will have to work with most students 10 to develop this skill over time. The following activities are well suited to developing skills in following directions: Identifying cause and effect relationships Summarizing main points after reading Locating and choosing appropriate reference materials Reading for personal learning Language Skill - Writing Using language accurately Organizing and relating ideas when writing Proofing and Editing Synthesizing information from several sources Documenting sources Developing an outline Writing to persuade or justify a position Creating memos, letters, other forms of correspondence Teamwork Taking initiative Working on a team Most of the activities are designed to be done and discussed in teams. The following activities are well suited to developing team interdependence skills: Thinking/Problem-Solving Skills Identifying key problems or questions Evaluating results Developing strategies to address problems Developing an action plan or timeline Earth/Environmental Science- Unit 3 DRAFT 11 Unit Guide: THE LITHOSPHERE Total: 15 - 90 min days OR 30 – 45 min days ENGAGE: Activating Prior Knowledge through journal entry about minerals: Ask students to answer these questions in a journal/log or generate answers in small groups and report out. (According to the NC Standard Course of Study, these topics/concepts were covered in the sixth grade (Goal 3) and eighth grade (Goal 4). http://www.learnnc.org/scos/2005-SCI/0006/05/) Suggested Journal Questions: What is an element? What are the major types of chemical bonding? Are you comfortable using the Periodic Chart of the Elements? Explain. What is density? What is the relationship between rocks and minerals? What do you already know about minerals? List your ideas. http://www.windows.ucar.edu/tour/link=/earth/geology/geology.html Time: 10 min ================================================================ EXPLORE: INTRODUCTORY ACTIVITY: SALT AND SAND IN WATER Focus Objectives: 1.01, 1.02, 1.04 Language (ELP) Objective for LEP students: Participate in a class discussion on what happens to salt and sand when mixed with water. Modifications for LEP students: Use this activity as a demonstration and explain each procedure as performed. Explain the terms settle and dissolve to assure student relate terms to the action of the sand and salt. Activity Time: 5 min (depending on discussion) Preparation Time: 2 min if done as a demo or 5 min. if students do it as a partner activity Earth/Environmental Science- Unit 3 DRAFT 12 Safety: none Notes: Use this activity as a catalyst to spark questions and generate interest. Students will observe that the sand (detrital sediment) settles to the bottom, unchanged, while the salt (chemical sediment) dissolves. Allow students to discuss their ideas. Do not get into the chemistry or mineralogy yet. The activities provided in this unit will help students develop an understanding. Guiding Question: What will happen to the salt and the sand when it is mixed with the water? Before the activity: The teacher should have students trained concerning safety procedures and movement when handling equipment such as gathering/disposing of water, sand, salt. After the activity: The teacher should acknowledge and accept all suggestions for the obvious conclusions and encourage students to state the obvious. Building trust and communication when discussing some obvious principals will allow students to discuss more difficult concepts later in the unit. ================================================================ EXPLAIN: BASIC MINERAL IDENTIFICATION Focus Objective: 2.01 Language (ELP) Objective for LEP students: 1. Reading a chart, classify minerals according to chemical and physical properties. 2. Describe in a chart the hardness, luster, color, specific gravity, and streak of minerals. 3. Explain to a partner different models of chemical structures that show mineral crystal formation. Modifications for LEP students 1. Model/illustrate each mineral characteristic before students use them to classify minerals. 2. Show examples, using minerals or other objects, to help LEP students visualize words used to describe luster. 3. Give students a list of minerals and their descriptions before the actual activity. Allow students to match minerals with their descriptions written on the list. Activity Time: 90 minutes Preparation Time: 45 minutes Earth/Environmental Science- Unit 3 DRAFT 13 Note: A student worksheet BASIC MINERAL IDENTIFICATION WORKSHEET is provided after the BACKGROUND INFORMATION which is located at the end of this document. You can modify this worksheet so it better suits your classroom and resources. Part five (Optical Properties) requires clear pieces of rhombohedral calcite and ulexite, thin pieces of mica, and polarizing filters. Make sure the specimens for determining specific gravity are small enough to fit in the graduated cylinders. If not, use beakers. Part 3 – Crystal Shape and Cleavage: Students are asked to distinguish between fluorite and quartz. This question could be applied to any mineral pairs that are difficult to distinguish. Guiding Question: How do scientists classify minerals based on scientific investigation? Before the activity: The teacher will need to prepare all the samples for testing and practice testing. Be sure to plan for enough space for students to move around the room through stations with each test set up with the equipment they need. Students should be familiar with safety issues and movement within your confines. After the activity: A discussion of their findings can take place in small groups or whole group. Students should be able to relate safety issues, comparison of mineral structures, and investigative measures to identify characteristics. ================================================================ EXPLAIN: Focus Objective: Activity Time: Preparation Time: Notes: Guiding Question: Before the activity: After the activity: ================================================================ ELABORATE: ROCK ROULETTE Earth/Environmental Science- Unit 3 DRAFT 14 Focus Objective: 2.03 Language (ELP) Objective for LEP students: 1. Draw and label the rock cycle to show the origins of sedimentary, igneous, and metamorphic rocks. 2. Explain to a partner the difference in extrusive and intrusive rocks and identify examples of each. 3. Complete a concept map using vocabulary. Modifications for LEP students: 1. Emphasize the most important vocabulary and reduce the quantity of vocabulary. 2. Model the process for moving from station to station for each activity. Elicit verbal response of understanding from students. 3. Pair LEP students with different proficiency levels for activities. 4. Create concept maps or graphic organizers to show vocabulary relationships. Activity Time: 30 min Preparation Time: 45 min Safety: none Notes: This activity provides an opportunity for students to examine the rock cycle and see it realistically – as a collection of interrelated systems. The developers identified eleven transitional stages through which earth materials might pass during the rock cycle. The term “earth materials” consists of all possible types of earth matter: sediment, the three types of rocks (sedimentary, metamorphic, igneous), lava, etc. There is a station for each of the components of the rock cycle. Materials might remain at a station briefly or for a very long period of time before eventually moving on to another stage. See additional ROCK ROULETTE set up information at the end of this document. Guiding Question: How do elements move through the rock cycle? Before the activity: The teacher needs to set up the area and make each station. Students need to be aware of how they will move through stations and work together to complete the task. After the activity: Students may be required to write an essay on their travels through the rock cycle or there may be group discussion on the event of their travels. . Earth/Environmental Science- Unit 3 DRAFT 15 ================================================================ EVALUATE: LOCATING PATTERNS OF EARTHQUAKE AND VOLCANO DISTRIBUTION Focus Objective: 2.04 Language (ELP) Objective for LEP students: 1. Read a chart to locate on a map the Ring of Fire 2. Explain to a partner the relationship between crustal movement and the occurrence of volcanoes and earthquakes. 3. Write a paragraph to describe the relationship between the locations of earthquakes and volcanoes. Modifications for LEP students: Begin the activity by accessing prior knowledge of mapping latitude and longitude. Review the ideas of plate tectonics and how plates move. After the activity relate the results of their map to plate boundaries. Activity Time: 90 min Preparation Time: 5 min Guiding Question: What is the worldwide pattern of Earthquake and Volcano Distribution? Before activity: The teacher should review how to plot points on a graph and how to use the map with respect to longitude and latitude. After activity: The teacher will lead the class in a discussion of their findings and the students should turn in their written explanations to the discussion questions. ================================================================ EXPLAIN: Pushing and Pulling of Mountain Building Focus Objective: 2.04 Modifications for LEP students: To assess prior knowledge ask students to draw and label large pictures of each type of plate boundary on newsprint. Students can show and explain their type of plate boundary to the class. Model the activity using key vocabulary needed by the students. Pair LEP students together to complete this activity. Earth/Environmental Science- Unit 3 DRAFT 16 Language (ELP) Objective for LEP students: Read activity directions to create mountains formed by different forces. Label a diagram using vocabulary relating to specific forces needed to create mountains. Activity Time: 40 min Preparation Time: 40 min Notes: Mountains and valleys are created by huge tectonic activity, or movement in the Earth. This movement occurs in many different ways, over millions of years. This lesson will show how mountains and valleys form as the result of tectonic forces. See Pushing and Pulling of Mountain Building following this section of the document for further information and the website. Guiding Question: How do tectonic forces build mountains? Before the activity: The teacher should attempt this activity beforehand and be aware of students who may be in special need of kinesthetic activities to ensure success. Be aware that students my need more space and that it might take more time depending on your students. After the activity: The teacher should be able to have a discussion on what was learned by each action taken on the materials and what each represented. ================================================================ ELABORATE: SLICE OF THE EARTH Focus Objective: 2.04 Language (ELP) Objective for LEP students: Read and follow directions to complete scale drawing. Orally share group scale drawing of layer of the Earth with the class. Participate in class discussion describing how scale models describe the layer of the earth. Write a paragraph describing the layers of the Earth Modifications for LEP students: Access prior knowledge of how to create scale drawings and remediate when necessary. Illustrate the concept of “drawing to scale” by using a map to show the distance from one city to another. Pair different English proficiency students together to complete the project. Earth/Environmental Science- Unit 3 DRAFT 17 Activity Time: 60 min Preparation Time: 20 min Safety: Beware of sharp objects. Notes: In this activity, students will construct a scale model of a “slice” of the earth. With this slice, students will illustrate the layers of the earth and their components. Additional information follows this section of the document. Guiding Question: How can the earth’s internal structure be inferred through the interpretation of seismic data? Before the activity: Students will need to understand procedures for moving around the room, getting supplies, and have some ideas of the formation of the earth. After the activity: Students will share their slices with the rest of the class and discuss the new concepts that they discovered. . ================================================================ EVALUATE: FINDING THE EPICENTER OF AN EARTHQUAKE Focus Objective: 2.04 Language (ELP) Objectives for LEP students: Compare and contrast P and S waves using a Venn diagram. Read and interpret data to create a earthquake wave travel-time graph. Orally explain to a partner how to determine the epicenter of an earthquake. Modifications for LEP students: Review with students the difference in the focus and the epicenter where they are located. Discuss P and S waves and how they move through the Earth with the students to access understanding before graphing the data and explain to the students the need for at least 3 data sites for determining the epicenter. Review how to create scale drawings and how to use a compass for the activity. Students will need to be aware of how to use the compass and the scale given on the attached map to graph larger distances. Earth/Environmental Science- Unit 3 DRAFT 18 Activity Time: 45 min Preparation Time: 5 min Safety: none Notes: In this investigation students will construct an earthquake wave travel-time graph. They will then use the graph to answer some questions about earthquakes. The complete instructions follow this section of the document. Guiding Question: What is an earthquake wave travel-time graph and how is it used? Before activity: Students should have an understanding of a seismograph and how it works to collect data. After activity: Students should be able to understand the importance of having 3 pieces of data from the graphs to accurately locate an epicenter. ================================================================ EXPLAIN: CHOCOLATE CHIP COOKIE MINING Focus Objective: 2.06 Language (ELP) Objective for LEP students: Participate in a class discussion on the economic impact of mining. Make a poster listing the environmental impacts of mining. Modifications for LEP students: When making posters, LEP students can draw and label illustrations to explain environmental impacts of mining. To help students make application of the environmental and economic impacts of mining ask them to find examples of mining in their country on the internet and bring them in to share. Earth/Environmental Science- Unit 3 DRAFT 19 Activity Time: 60 min Preparation Time: 30 min Notes: This is a mining game using edible cookies and calculating the costs. Students will be tempted to eat the cookie before the complete the mining exercise. The complete instructions follow this section of the document. Guiding Question: What is the economic importance and impact of developing economical mining practices? Before the activity: Game pieces and cookies will need to be purchased along with explanation for rules that will keep students working collaboratively. After the activity: Students should discuss their results then apply them to research on existing mining operations. ================================================================ APPENDIX Background information for MINERAL IDENTIFICATION: HARDNESS is the ability of a mineral to resist being scratched. Students will determine the hardness of selected minerals using the “hardness kit.” Remind students to use common sense and not scratch a soft mineral with a hard object. The key is to observe which is actually DOING the scratching and which is BEING scratched. Rub the mineral against another known mineral or object to see if it will become scratched. The chart below shows how you might test a mineral for hardness using a combination of Moh's hardness scale and field hardness scales. Talc, the softest mineral, is assigned a value of 1, while diamond, the hardest is assigned the value of 10. A mineral with a higher Moh hardness value can scratch a mineral with a lower Moh hardness value. A mineral may be scratched by any of the items corresponding with a higher hardness value. When testing for hardness, remind students to start with the softer mineral or item and work up from there. For example, an unknown mineral, which is soft, should be scratched with your fingernail first, working your way up through the harder minerals or items until you find the hardness. In your instructions, let the students know which “unknown” minerals are to be tested for hardness. Whether a mineral is harder or softer than glass is a property thoroughly ingrained in geology. There is less error involved than when using minerals and other items. HARDNESS SCALE (Moh's and Field Hardness Scales) HARDNESS MINERAL HARDNESS TEST ITEM 1 talc Fingernail will scratch it Cannot scratch glass 2 gypsum Fingernail will scratch it Cannot scratch glass 3 calcite Penny will barely scratch it Cannot scratch glass 4 fluorite Penny will scratch it Cannot scratch glass 5 apatite Will scratch penny; cannot Cannot scratch glass Earth/Environmental Science- Unit 3 DRAFT 20 scratch glass 6 7 8 9 orthoclase feldspar quartz topaz corundum 10 diamond Can barely scratch glass Will scratch glass Will scratch glass well Will put deep incision in glass Will scratch all of the above Used to cut glass SPECIFIC GRAVITY: Each mineral has its own value or range of values for specific gravity, which is the density of the mineral compared to the density of water (1 gram per cubic centimeter or 1 g/cc) The specimens used in this section should be small enough to fit in your graduated cylinder. If a graduated cylinder is not available, a beaker may be used, however, there will be a larger margin of error without the accuracy of the graduated cylinder. If beakers are being used to find volume, larger specimens may be used. Small cubes of hematite and galena, as well as native copper work well for this activity. Step one: Find the mass of the mineral using the scale or balance. This should be done first, before the mineral gets wet. Step two: Find the volume of the mineral by determining the volume of water that gets displaced. If a mineral with all right angles between sides is available (i.e pyrite), students can determine the volume by measuring the sides. This result can be compared with the one derived by the displacement of water. Step three: Calculate the specific gravity (mass divided by volume) and compare results with the actual specific gravity listed in the rock and mineral guidebook. COLOR: Color is easy to observe, but is not always a reliable characteristic for the identification of minerals. A mineral may come in a variety of colors or change due to environmental conditions. LUSTER: A mineral's luster describes the way light is reflected from its surface. Examples of luster include - metallic, nonmetallic, brilliant (adamantine), glassy, greasy, pearly, or silky. Pyrite and galena have metallic lusters. Quartz and fluorite have glassy lusters. STREAK: The streak of a mineral is the powder left behind when the mineral is rubbed onto a streak plate. It is the color of the mineral in a powdered form. As in the case of pyrite, a mineral’s streak may be different from the color of the mineral itself. The softer minerals (less than 7) generally leave behind a streak on a streak plate. CLEAVAGE OR FRACTURE: These two characteristics describe the way a mineral breaks. If a mineral exhibits a fracture, the breakage is uneven and rough. Glass, for example, does not break evenly, rather it fractures. Cleavage means to break along a smooth, defined plane. It can occur in only one direction, i.e. mica or in as many as 4 directions, i.e. fluorite. A mineral specimen may have one or more smooth, defined surfaces. This does not necessarily mean that the mineral exhibits cleavage. For example, a quartz crystal may have smooth, defined surfaces, Earth/Environmental Science- Unit 3 DRAFT 21 however, if it breaks, will shatter like glass (fracture). The smooth, outside surfaces are the actual crystal faces, which had formed when the mineral finished growing. Distinguishing between crystal faces and cleavage comes with experience. CRYSTAL SHAPE: When the atoms come together to form a mineral, a pattern of planar surfaces often develops on the outside which is the mineral’s crystal shape. This is often difficult to determine when identifying minerals. The following link discusses crystallography in detail. It is a good resource for advanced students. http://webmineral.com/crystall.shtml CRYSTAL HABIT: Crystal Habit is the general size and shape (or appearance) of the mineral. The following link illustrates many of the known crystal habits http://www.galleries.com/minerals/property/habits.htm Crystal habit and crystal shape are terms that can be confusing to students. Mineralogists often use “crystal form” to describe the situation when the outward appearance of a mineral takes on a regular geometric shape. Crystal habit is related to how a mineral breaks, whether it has a regular geometric shape or not. MINERAL CLEAVAGE MINERAL sphalerite fluorite calcite halite galena # OF CLEAVAGE DIRECTIONS 6 4 3 3 3 ANGLE OF CLEAVAGE DIRECTION Not 90 degrees Not 90 degrees Not 90 degrees 90 degrees 90 degrees muscovite 1 flat BASIC MINERAL IDENTIFICATION WORKSHEET Name: ___________________ You will be working with 16 different minerals (listed below). Your job will be to identify the minerals based on their physical properties. Each mineral has a number. The minerals you will be working with are listed in a scrambled order below. The minerals with an asterisk (*) after their name are the ones for testing hardness. Your teacher will tell you the numbers of the hardness minerals in your kit. Write those numbers in the left column of the data table (below) ____ PYRITE ____ TALC* ____ HEMATITE Earth/Environmental Science- Unit 3 DRAFT 22 ____ CORUNDUM * ____ COPPER ____ MAGNETITE ____ MUSCOVITE ____ KAOLINITE ____ GALENA ____ HALITE ____ CALCITE * ____ QUARTZ * ____ ORTHOCLASE * ____ FLUORITE * ____ BORNITE ____ GYPSUM * PART ONE – HARDNESS Your instructor will tell you the numbers of the minerals used for the hardness tests. Write these numbers in the column on the far left. Hardness of objects: fingernail is 2.5; copper penny is 3; glass is 5.5 Identify the mineral quartz. This mineral is one used in the following hardness test. Use the following questions as guide for each mineral you test. Write “Yes” or “No” in columns 2-5. Can the mineral be scratched by your fingernail? Can the mineral be scratched by a penny? Is the glass plate harder than the mineral? Is quartz harder than the mineral? MINERAL FINGERNAIL PENNY GLASS PLATE Yes or No Yes or No Yes or No Yes or No QUARTZ quartz gypsum orthoclase corundum talc calcite fluorite HARDNESS SCALE: According to your data write the hardness scale (1-10) 1. ____________________ Earth/Environmental Science- Unit 3 6. ____________________ DRAFT 23 2. ____________________ 7. ____________________ 3. ____________________ 8. Topaz 4. ____________________ 9. _____________________ 5. Apatite 10. Diamond + When doing the scratch test, it is important to observe what is actually being scratched and what is doing the scratching! How could you tell? PART TWO - STREAK Two of your minerals have characteristic streaks, which is the powder left behind on a non-glazed porcelain streak plate. The two minerals are pyrite and hematite. What color is the streak for each mineral? Pyrite is mineral # _____ and has a ___________________ streak. Hematite is mineral # _____ and has a ___________________ streak. PART THREE - CRYSTAL SHAPE AND CLEAVAGE Inspect the minerals in your collection. Some of them appear to have been broken unevenly, or fractured, while others appear to have been broken along specific planes and at specific angles. The minerals in the latter group exhibit cleavage. List four minerals that appear to have cleavage and determine how many directions or planes of cleavage exist. MINERAL # NAME OF MINERAL Earth/Environmental Science- Unit 3 DESCRIPTION OF CLEAVAGE DRAFT 24 Quartz and fluorite can look very similar if cleavage and fracture are not readily apparent. Both come in a variety of colors. What other test can be used to distinguish, without a doubt, the quartz from the fluorite? PART FOUR – LUSTER Luster describes how the mineral reflects light. Write the term which best describes the luster of each mineral. The most common terms used to describe luster are as follows: vitreous metallic earthy silky PYRITE __________________________ pearly adamantine HEMATITE ________________________ TALC ____________________________ CORUNDUM ______________________ BORNITE _______________________ KAOLINITE ________________________ HALITE _________________________ QUARTZ _________________________ FLUORITE ______________________ GYPSUM __________________________ COPPER _________________________ MUSCOVITE ______________________ GALENA _________________________ CALCITE __________________________ FELDSPAR _______________________ BORNITE _________________________ PART FIVE - OPTICAL PROPERTIES Materials: clear rhombohedral calcite specimen, clear ulexite specimen, thin pieces of muscovite, polarizing filters, scotch tape (the old-fashioned clear kind) 1) Place your piece of clear ulexite over printed words and look through it. Describe what you see. 2) Place your piece of clear calcite over printed words and look through it. Describe what you see. 3) Peel a thin sheet of muscovite and look at a light source through it. Describe what you see. Earth/Environmental Science- Unit 3 DRAFT 25 4) Place the muscovite between two polarizing filters. Hold it up to the light source while spinning the polarizing filters. Describe what you see. PART SIX – SPECIFIC GRAVITY Materials: 100 mL graduated cylinder, scale, samples of galena, pyrite and copper (small enough to fit inside graduated cylinder), rock and mineral guidebook. FYI: The most common unit for the density of solids is grams per cubic centimeter (g/cc) This translates to grams per milliliter for liquids (g/mL). The average density of water = 1 g/mL = 1 g/cc Procedure: 1) Select one of the designated samples. Determine the mass of the specimen. Record the mass on the data table. 2) Pour water into the graduated cylinder up to the 50mL mark 3) Measure the volume of the sample by carefully dropping it in the graduated cylinder. Record the amount of displacement in mL 4) Calculate the specific gravity by the following formula: mass / volume 5) Compare your calculated specific gravity with the actual specific gravity as shown in your rock and mineral guidebook. Mineral Mass (g) Volume (mL) Earth/Environmental Science- Unit 3 Specific Specific gravity gravity from (mass/volume) field guide DRAFT 26 ROCK ROULETTE Teacher Notes: Ease of preparation is difficult for first time set-up. We recommend laminating and saving materials as a kit for future use. This activity was developed by Stan M. Schmidt and Courtney Palmer. It provides an opportunity for students to examine the rock cycle and see it realistically – as a collection of interrelated systems. The developers identified eleven transitional stages through which earth materials might pass during the rock cycle. The term “earth materials” consists of all possible types of earth matter: sediment, the three types of rocks (sedimentary, metamorphic, igneous), lava, etc. There is a station for each of the components of the rock cycle. Materials might remain at a station briefly or for a very long period of time before eventually moving on to another stage. Each station has a six-sided die, which is marked according to the probability of what happens to the earth material at that stage. The station “Igneous Rocks” is used as an example and the six-sided die at this station is marked in the following way: two sides are marked weathering and erosion,” two sides are marked “high temperature and pressure,” and the last two sides are marked “melting.” In other words, there is a 33.33% chance that igneous rock will weather and erode, a 33.33% chance that the rock will be subjected to high temperature and pressure, and a 33.33% chance that the rock will melt. The probability closely matches what happens in the “real world.” A student rolls the die and goes to the station indicated by that role. The developers suggest the stations are arranged in a large circle. A sign is placed at each station to go along with that station’s specific six-sided die. Let students know that they are to think of themselves as particles of soil or mineral grains in rock moving through the rock cycle. Instruct students to draw a map of the stations in their notebooks and identify their starting point station. Students are to record their movements through the stations. This can be done by drawing arrows that trace their moves. Since some of the sides of the die at various stations are marked “stay where you are,” students should also keep a record of how long they stay at a particular station. This can be done using tally marks. Each step of the rock cycle may take as little as 200,000 years or as much as several million years. Each roll of the die has been defined as 200,000 years. Students can multiply the total number of dice rolls to calculate the total time of their pattern from beginning to end. The developers recommend 20 minutes to maximize student involvement an interest. The eleven die should be constructed with heavy construction paper so they can be reused many times. Below is a list of stations and how each station’s die should be labeled: NAME OF STATION STATION 1: COMPACTION AND # OF DIE SIDES MARKED WITH GIVEN “GO TO” OPTION 3 3 Earth/Environmental Science- Unit 3 GO TO: Sedimentary rock Compaction and cementation DRAFT 27 SEDIMENTATION STATION 2: HIGH TEMPERATURE AND PRESSURE STATION 3: SEDIMENTS STATION 4: IGNEOUS ROCKS STATION 5: TO THE SURFACE STATION 6: METAMORPHIC ROCK STATION 7: SEDIMENTARY ROCK STATION 8: MELTING STATION 9: COOLING AND HARDENING (CRYSTALLIZATION) STATION 10: MAGMA STATION 11: WEATHERING AND EROSION 3 3 2 4 2 2 2 4 2 2 2 2 2 2 2 3 3 3 3 2 4 3 3 (stay where you are) Metamorphic rock High temperature and pressure (stay where you are) Compaction and sedimentation Sediments (stay where you are) Weathering and erosion High temperature and pressure Melting Weathering and erosion To the surface (stay where you are) Melting To the surface High temperature and pressure High temperature and pressure Melting Weathering and erosion Magma Melting (stay where you are) Igneous rock Cooling and hardening (stay where you are) Cooling and hardening Magma (stay where you are) Sediments Weathering and erosion (stay where you are) LOCATING PATTERNS OF EARTHQUAKE AND VOLCANO DISTRIBUTION Problem: What is the worldwide pattern of Earthquake and Volcano Distribution? Materials: A world map showing latitude and longitude (on back) Four different colored pencils Procedure: 1. Use the information in the table to plot the location of each earthquake. Use one of the color pencils to label on the world map each earthquake location with the letter E inside the circle. 2. Use another color pencil to plot volcano locations and place the letter V inside the circle. 3. Using a 3rd color, lightly shade the areas in which earthquakes are found. 4. Using a 4th color, lightly shade the areas in which volcanoes are found. 5. Then answer the questions that follow. Key: Earthquake Color Earth/Environmental Science- Unit 3 Volcano Color DRAFT 28 Earthquake Zones Volcano Zones Define the following terms from the notes: Magma, Volcanism, Lava, Vent, Crater, Volcano Observations: Answer the following questions. 1. 2. 3. 4. 5. 6. Are earthquakes scattered randomly over the surface of the Earth or are they concentrated in definite zones? Are volcanoes scattered randomly over the surface of the Earth or are they concentrated in specific zones? Are most earthquakes and volcanoes located near the edges of continents or near the center of continents? Are there any active volcanoes near your home? Have there been any earthquakes near your home? Discussion: In 2-3 sentences, describe any patterns you observed in the distribution of earthquakes and volcanoes. Describe the relationship between the locations of earthquakes and volcanoes. PUSHING AND PULLING MOUNTAINS http://www.nps.gov/brca/forteachers/landformact4.htm Summary: Mountains and valleys are created by huge tectonic activity, or movement in the Earth. This movement occurs in many different ways, over millions of years. This lesson will show how mountains and valleys form as the result of tectonic forces. Instructional Method: Activity Goal: To experiment with how tectonic forces form mountains. Objectives: Students will be able to: Recreate each landform made by drawing it on paper or using the foam strips Perform each force needed to create different landforms Label on paper the direction of the movement of the rock corresponding to each force Locate these landforms in nature or in photos Time: Set up: 40 min. Experiment: 20 min. Cleanup and discussion: 20 min. Materials Needed: Earth/Environmental Science- Unit 3 DRAFT 29 Foam of different thickness and color (craft and camping pad foam) String Rope 4x4 piece of wood 2 feet long Saw Scissors Vocabulary: basin batholith fault fold graben hill horst igneous intrusion laccolith mountain normal fault tectonic thrust fault valley Background: Mountains and valleys form in many ways. Two types of tectonic forces can result in mountains and valleys: compression (squishing) and extension (pulling apart). These forces commonly associate with mountain building but also form valleys. Compressing continents occur when two continents collide. In crustal collision, the buckling action of rock is similar to what happens to the hoods of cars in a head-on collision. The buckles form mountains and valleys. In rock the buckles can fracture and slide on top of each other, causing that area to increase in thickness. An example of two continents compressing into each other resulted in the Himalayan Mountains. This happened when India collided with Asia millions of years ago. They are still compressing into each other and the mountains are still growing today. An older example of the same thing happening in the United States is the Appalachian Mountains. North America and Africa collided about 200-300 million years ago forming this great mountain system. They are no longer growing but diminishing due to erosion over time. Great Smoky Mountains National Park is located in the heart of the Appalachians. Earth/Environmental Science- Unit 3 DRAFT 30 Stretching a continent causes it to thin and break in the same way silly putty breaks when it is cold and stretched quickly. Faults form throughout the stretched portion of the crust and allow large blocks of solid rock to move up or down as dictated by magma movement in the mantle. These massive blocks of rock are called fault blocks. Fault blocks are divided into two categories: horsts and grabens. A horst is an uplifted fault block and a graben is a fallen or down dropped fault block. A geographic region in the western United States known for horst and graben fault block structures is the Basin and Range. This region begins at Zion National Park and extends into eastern California. A national park sharing the region's name and located within the Basin and Range is Great Basin National Park. The Plateau region in the Four Corners area of the American southwest (southern Utah, northern Arizona, southeastern Colorado and northwest New Mexico) may have uplifted by the collision of two tectonic plates. However, this area did not get mangled and buckle like the Appalachian Mountains. Buckling occurred close to the plate contacts forming the Sierra Nevada mountains. The Pacific Plate is thought to have slid under the American plate for a long distance until it sunk deep into the mantle. Once it was deep enough, it melted and the melted rock rose towards the surface. Rising magma uplifted the entire area forming the Colorado plateau. Much of the rising magma remained below the flat-lying crust. Laccoliths and batholiths formed in locations on the Colorado Plateau where the magma did not break through the surface. These two landforms are like "blisters" of magma under the rocks. Eventually, the magma cooled and hardened forming a solid core of igneous rock. These igneous "blisters" exposed at the surface form batholiths and laccoliths. Batholiths are igneous intrusions with irregular shapes, top and bottom. Laccoliths are rounded on top and have a flat bottom. Scientists believed that because the igneous intrusion was pushed into flat lying horizontal layers the bubble's bottom spread along a bedding plane, giving it a flat bottom. These blisters form the Henry mountains, Manti-La Sal mountains, San Francisco mountains and Navajo Mountain, among others. Each one of these mountain ranges can be seen from surrounding national parks; Arches National Park, Bryce Canyon National Park, Capitol Reef National Park, Canyonlands National Park and Hovenweep National Monument. Many times a combination of extension and collision is responsible for the creation of landforms. Instructional Procedures: 1. Instructional Procedures for Creating Activity Tools: 1. Cut foam into strips 2-4 inches wide and 8-12 inches long. 2. Cut two small holes into each end with scissors or a paper punch. Earth/Environmental Science- Unit 3 DRAFT 31 3. Alternate thickness and color to create a stack of 3 to 5 pieces. 4. Thread string through the holes cut in the foam to fasten the pieces together forming a foam sandwich. 5. Make sure the string is loose enough that the foam pieces can slide when bent. 6. Cut wood into angled pieces of 45 degrees or more. 7. Drill a hole through each piece so they line up. 8. Slide a rope through the blocks and tie a knot at each end. Make sure to leave 3 to 4 inches between the knot and the end of the block. 2. Activity Instructions: 1. The foam layers represent layers of rock. By pushing and folding the foam you can imagine how rock layers respond to the same forces. Obviously, folding and buckling mountains in nature takes a very long time, but the process is replicated quite well with foam. 2. Wood blocks represent portions of the crust as it responds to extensional forces. Start with the blocks pushed together forming a flat surface and then pull them apart to form mountains and valleys. In nature, the higher portions are called horsts and the falling portions are called grabens. 3. Explain what the layers of foam represent and what the wood blocks represent. If possible show pictures of landforms that show the rock layers with folds or bends. A topographic or geologic map of Nevada would be helpful to show the mountains and valleys formed by stretching the crust. 4. Demonstrate to the class the different types of mountain building processes. 5. Give a demonstration on how they can use each tool to build their own mountains. 6. Break students into as many groups as possible so that each group can have their own foam and blocks. 7. Recreate each landform they have studied. 8. Draw on a paper the forces, noted by arrows, that are responsible for creating each landform. Discussion: Earth/Environmental Science- Unit 3 DRAFT 32 What are the different forces that build mountains? How do they work? Are there any mountains near you? If so, do you know how they formed? Can we ever feel mountains moving (yes, earthquakes)? What do we call the shaking we feel when there is movement along a fault? Variation: If foam is not available, try using short stacks of different color paper. Each stack of paper should represent one rock layer. Stacks should be at least 1/8 inch thick and no more than 1/2 inch thick. Each stack should be a different color. Follow steps 2-5 of procedures for building tools in order to create paper rock layers, then proceed with activity. Extension: Have students hypothesize what may happen if forces were placed in different locations or from only one side, etc. Write down the hypotheses. Perform the hypotheses on the foam layers and blocks. Write down what happened to the foam and blocks ( i.e. what landforms they created). SLICE OF THE EARTH Essential Question: How can the earth’s internal structure be inferred through the interpretation of seismic data? In this activity you will construct a scale model of a “slice” of the earth. With this slice, you will illustrate the layers of the earth and their components. Materials: two 11 x 17 inch sheets of paper meter stick ~ 70 cm long string with loop at one end tape pencil Procedure: 1. Tape the two sheets of paper together at the short end to make one sheet that is 11 x 34 inches. 2. Draw a light center line and “width lines” on the 11 x 34 paper as short dashed lines to construct a scale model of a slice of the earth’s interior (like a pizza slice). The scale is 1:10 million of 1 cm = 100 km. This results in a slide with a Earth/Environmental Science- Unit 3 DRAFT 33 3. 4. 5. 6. 7. radius of 63.7 cm which corresponds to the earth’s actual radius of 6371 km. Using the dimensions shown in Figure 1 and Table 3, construct your slice. Draw the surface arc line by using the string with a loop and a pencil with the length from the center point to the pencil in the loop being 63.7 cm. Draw the angle line using information from Table 3. Now erase all light pencil lines. Complete your scale model slice by drawing arcs at the appropriate radii corresponding to the Moho, Lithosphere-Asthenosphere, Transition Zone, CoreMantle, and Outer Core-Inner Core boundaries as given in Table 1 and illustrated on Figure 2. Label the boundaries and layers of the earth and color the various layers. Once your slice is complete, stick it on the wall so that as a class you will create a scale model of the whole earth and its layers. Questions: Answer the following questions in complete sentences. 1. What does it mean to make a “scale” model of the earth? 2. What would our classroom earth look like if you did not make your slices to scale? 3. Describe how this activity simply describes the layers of the earth. 4. What observations have scientists made that tell us about the different layers of the earth? 5. Is the earth really divided into layers that have distinct boundaries and are uniform throughout the entire earth? Describe why or why not. Table 1: Measurements for Slice of the Earth Actual Value Scale Value (1:10 million Scale) 6371 km 63.7 cm Depth* to base of the crust (average) 35 km 0.35 cm Depth* to base of lithosphere (average) 100 km 1.0 cm Depth* to base of upper mantle 670 km 6.7 cm Depth* to core-mantle boundary 2885 km 28.9 cm Depth* to outer core-inner core boundary 5155 km 51.6 cm Radius of Earth *Measure downwards from the surface after drawing the arc representing the surface at a distance of 63.7 cm from the Earth’s center and drawing the diagonal lines completing the “pie-shaped slice” of the Earth. Table 3: Measurements of Width Lines to Produce Slices of Different Angles for Different Class Sizes. Earth/Environmental Science- Unit 3 DRAFT 34 Angle of Slice (degrees) Number of Slices (Class Size) to Make a Full Circle Distance From Center Line to Width Line (see Figure 1) 12 30 6.7 cm 15 24 8.3 cm 20 18 11.1 cm 24 15 13.2 cm Figure 1. Dashed lines are light pencil lines (draw first) that can be erased after the solid lines of the "slice" are drawn. Figure 2. Earth's interior structure. Earth/Environmental Science- Unit 3 DRAFT 35 Earth/Environmental Science- Unit 3 DRAFT 36 FINDING THE EPICENTER OF AN EARTHQUAKE Background Information: Movement within the earth along a fault or between plates causes seismic shock waves to spread out in all directions. The point at which the movement occurs is called the focus. Above the focus, on the surface of the earth is the epicenter. There are two distinct types of seismic waves that are used to determine an earthquake’s epicenter: P-waves (primary waves) move in a push and pull fashion, travel the fastest and arrive at seismic stations first. S-waves (shear waves or secondary waves) move in a side-to-side fashion, travel slower than P-waves and arrive at a seismic station after the P-waves. Each type of wave is affected by the density of the earth material in which it travels. P-waves can travel through both the solid crust and mantle and the molten outer core. S-waves can also travel through the solid parts of the earth, but not through liquid or molten material. Seismic stations throughout the world can detect distant earthquake waves. The key is to determine, from the seismograph reading, the time lapse between the arrival of the first P-wave and the first S-wave. A seismologist can then use data from multiple seismic stations to determine an earthquake’s epicenter. Data from three stations are necessary to do this. When seismograph data from stations at three different locations is compared, the precise location of an earthquake’s epicenter can be determined. Materials: Drawing compass with pencil Graph that plots distance to epicenter (X-axis) and difference in arrival time between P and S waves United States map Procedure: 1. Examine the three seismograms and record the time difference in minutes between the arrival of the first P-wave and the arrival of the first S-wave. Do this for each seismic station. Earth/Environmental Science- Unit 3 DRAFT 37 2. Use the Travel-Time graph to determine the distance in miles each station is from the epicenter of the earthquake. To do this, find the time in minutes (Y-axis) and go horizontally across to the curve. Now go vertically downward to the X-axis and record the corresponding distance in miles. 3. Use the distance from the epicenter in miles for the first seismic station. Place the compass point exactly on the “0” mark of the scale. Widen the compass so the pencil point matches the distance. 4. Maintaining the compass width, place the point of the compass on the map at the location of the first seismic station. Sketch a complete circle. 5. Repeat steps 2-4 for the remaining two seismic stations. 6. The three circles should intersect at a point. This is the location of the earthquake’s epicenter. Seismograph Station Arrival (clock time) “P” wave “S” wave Difference in arrival time min & sec Distance to epicenter Oklahoma City Earth/Environmental Science- Unit 3 DRAFT 38 Denver Tampa Chicago Earth/Environmental Science- Unit 3 DRAFT 39 Earth/Environmental Science- Unit 3 DRAFT 40 CHOCOLATE CHIP COOKIE MINING Background: Rich deposits of minerals from which valuable metals can be recovered profitably are called ores. If an ore is to be economical (profitable to mine considering the costs of extraction, transport and selling price) it must have a certain level of concentration. The mining industry has practical concerns: How long until the mineral “reserve” is exhausted? Are there yet-to-be-discovered deposits out there? When higher grade deposits are mined out, is it feasible to rely on lower-grade deposits, which are often more costly to recover? Following the procedure are descriptions of the various types of mineral deposits. Materials: several varieties of chocolate chip cookies (high, medium and low-end) paper money (tens, fives and singles) grid paper toothpicks (flat and round) paper clips Procedure: 1) Each player starts with $19.00 of play money, a Cookie Mining sheet, and a sheet of grid paper. 2) Each player must buy his/her own mining property, which is a cookie. Only one “mining property” per player. Players can choose from three different types of cookies. The most desirable cookie (and the most expensive) has the largest (or most) chocolate chips. PRICES OF MINING PROPERTY AND EQUIPMENT: Low-end cookie $3.00 Medium grade cookie $5.00 High-end cookie $7.00 flat toothpick $2.00 round toothpick $4.00 paper clip $6.00 3) After the mining property (cookie) and mining equipment are bought, the player places the cookie on the grid paper, and, using a pencil, traces the outline of the cookie. The player must count each square that falls inside the circle. Put together partial squares to make full squares. 4) While mining the cookie (removing the chips from the cookie) use only the mining tools. Each student should time his/her operation. Mining costs are $1.00 per minute and this will be factored in to the spreadsheet. 5) When mining ceases (allow a maximum of five minutes), each student should record the total time spent mining the cookie. 6) After the cookie has been “mined,” the cookie should be placed back into the circled area on the grid paper. This can only be accomplished using the mining tools – No fingers or hands allowed! Reclamation costs are $1.00 per square over the original count. 7) The chips (ore) should be separated so they can be redeemed for cash. Sale of a chocolate chip mined from a cookie brings $2.00 (broken chocolate chips can be combined to make one whole chip). RULES Only the mining tools and the paper may touch the cookie. Earth/Environmental Science- Unit 3 DRAFT 41 Players should allow a maximum of 5 minutes for mining. Mining may be completed before the five minutes are used up. Students are charged only for the amount of time used in mining ($1.00 per minute). A player can purchase as many mining tools as the player desires, and the tools can be of different types. If a mining tool breaks, they are no longer usable and a new tool must be purchased. COOKIE MINING SPREADSHEET 1. Name of mine: __________________________________________ 2. Price of mine: ___________ 3. Size of mine: ______ squares covered 4. Equipment: Flat toothpick ____ x $2.00 = ___________ Round Toothpick ____ x $4.00 = ___________ Paper clip ____ x $6.00 = ___________ 5. Mining: _____ minutes x $1.00 = ___________ 6. Reclamation: ______ squares x $1.00 = ___________ TOTAL COST OF MINING = ______________ 7. Chip removal: _____ chips x 2.00 per chip VALUE OF ORE MINED = _______ MONEY LEFT OVER FROM BEGINNING = _______ NET GAIN OR LOSS FROM MINING (Value of ore + money left over from beginning) – Total cost of mining = Earth/Environmental Science- Unit 3 DRAFT 42 TYPES OF MINERAL DEPOSITS HYDROTHERMAL DEPOSITS These deposits form in hot aqueous solutions. Vein deposits (also called “veins”) are tabular or sheet-like. Ores may be found in veins or in adjacent country rock Examples of vein ores: pyrite (FeS2), galena (PbS), sphalerite (ZnS), cinnabar (HgS), covelite (CuS) and chalcocite (CuS) Hydrothermal solutions reaching the Earth’s surface appear as hot springs and geysers. Precipitated mineral ores (pyrophyllite and sericite) from ancient hot springs and geysers occur in Hillsborough, NC. DISSEMINATED DEPOSITS These deposits are scattered throughout much larger volumes of rock, rather than being concentrated in veins. Porphyry copper deposits can be found in the American Southwest and Chile as well as other places. Igneous intrusions may occur in numerous scattered fractures over a large volume of rock. Hydrothermal solutions may penetrate cracks and precipitate ore minerals over a large volume of rock. Open pit copper mines near Tucson, AZ produce chalcopyrite, malachite and chalcocite. A lot of replacement deposits such as skarns are massive and disseminated. Carbonates dissolved by hydrothermal solutions and replaced with sulfides produce minerals such as galena and sphalerite. IGNEOUS ORE DEPOSITS Minerals crystallize from molten magma, settle and accumulate on the floor of a magma chamber – ex. Cr and Pt ores in S. Africa and Montana. Bushveldt complex – South Africa – dark chromite layers PEGMATITES Coarse-grained, intrusive rocks (granitic) found in veins, dikes and lenses in granitic batholiths. and formed by fractional crystallization of granitic magma. As magma cools, the last melt to freeze solidifies as a pegmatite where minerals present in trace amounts are concentrated. Rare mineral deposits rich in boron, lithium, fluorine, Niobium, Uranium. Examples of pegmatite gemstones: emerald, tourmaline. Unusually large mineral grain size is found in some pegmatites. KIMBERLITES One of the most valuable minerals, diamonds, originates chiefly in ultramafic igneous rocks called kimberlites, named for Kimberley, South Africa, where they are found in relative abundance. Rocks forcefully intruded to surface from deep in the crust and upper mantle in the form of a long, narrow pipe. Diamonds (and other minerals found in kimberlite pipes) can only be formed under the conditions of extremely high pressure that exist at such depths. Kimberlites erupt to the surface at high speeds, propelled by pressurized volatiles such as water and carbon dioxide. No one has ever seen a kimberlite eruption. Diamonds and other kimberlite minerals have been found long distances from their pipe of origin, carried by erosional agents (surface water, glaciers) SEDIMENTARY MINERAL DEPOSITS Include some of the world’s most valuable mineral sources. Many economically important Earth/Environmental Science- Unit 3 DRAFT 43 minerals segregate by chemical and physical means as an ordinary result of sedimentary processes. (see chapter 7 – sedimentary rocks) Non-metallic sedimentary minerals include: Limestones – chemically precipitated by marine organisms (cement, agricultural lime, building stone) Pure quartz sands – left behind due to resistance to weathering (glassmaking, fiber optics) Coarse sands and gravels – abundantly distributed by Pleistocene glaciers in many areas of the northern U.S. and Canada (construction purposes) Clays of high purity – prolonged weathering of feldspars (ceramics in both home and industry) Evaporite deposits – separated from seawater by precipitation Gypsum (plaster of paris), Sodium and Potassium salts (table salt, fertilizer), Phosphate rocks – marine shales and limestones enriched in phosphate by chemical reaction with deep seawater (raw material for the world’s fertilizer industry) Metallic Sedimetary minerals – important sources of copper, iron and other metals. Chemically precipitated in sedimentary environments to which large quantities of metals were transported in solution. Kupferschiefer beds “copper slate” of Germany are Permian in age. May have precipitated from hot brines of hydrothermal origin, rich in metal sulfides, that interacted with sediments on the ocean bottom. Banded iron formations - Major iron ores have been found in Precambrian sedimentary rocks. At that time in the Earth’s history, the atmosphere was poor in oxygen. This allowed an abundance of iron in its soluble (lower oxidation state or ferrous – Fe2+) form to be leached in great quantities from the land surface. The ferrous iron was transported in solution by groundwater to broad, shallow marine environments where it could be oxidized to its insoluble ferric form (Fe3+) and precipitated. In many of these basins, iron was deposited in thin layers alternating with layers of siliceous sediments (called “cherts”). Such iron ores are called “Banded iron formations.” In the U.S., the Lake Superior iron deposits are of this type. PLACERS Many rich deposits of gold, diamonds and other heavy minerals such as magnetite and chromite are found in placers; ore deposits that have been concentrated by the mechanical sorting action of river currents. The heavier minerals tend to accumulate on river bottoms and sandbars, where the current is strong enough to keep the lighter minerals suspended and in transport, but too weak to move the heavier mineral. In a similar manner, ocean waves preferentially deposit heavy minerals on the beach or on shallow offshore bars. Inspect “heavy sand” samples (bags of illmenite sand and/or sand composed of minerals containing manganese) from the inner coastal plain of Virginia. Some placers can be traced upstream to the location of the original mineral deposit, usually of igneous origin, from which the minerals were eroded. The “Mother Lode,” an extensive gold-bearing vein system in the western flanks of the Sierra Nevada batholith (large igneous intrusion) produced the placers that were discovered in 1848 and lead to the California Gold Rush. Examine the publication – “Gold Deposits in North Carolina.” The nation’s firstever gold rush occurred at the turn of the century (~ 1800) near Charlotte after a gold nugget was found by a teenager, Conrad Reed. Earth/Environmental Science- Unit 3 DRAFT 44 Goal 2.01 Activities (*** - 90 min days) MINERAL FAMILIES AND FORMATION Guiding Question: How are the physical properties of minerals dependent on their bonding formation? Teacher Notes Supplementary Notes Notes: If you have a fairly substantial mineral collection and many of your students have already taken chemistry, this activity is a nice extension for understanding and developing an appreciation for how minerals form. Prepare for this multiple-day activity by organizing your mineral collection according to mineral families. Common mineral families include: the silicates, the carbonates, the oxides, the sulfides, the sulfates and the halides. There are numerous smaller mineral families. Activity time: 90 min Before Activity: gather minerals and be proficient in the mineral families. Review with students how the notebook should be organized. AD CAMPAIGN FOR A MINERAL Guiding Question: After activity: have students create posters to share with the class or lead in a group discussion of their findings. Collect the notebooks for grading. Notes Challenge the students to design an ad campaign for a mineral, which will appear on a large highway billboard or on two pages of a popular magazine. You could also give students the option of doing a skit, a radio ad or television/info commercial. The product should highlight one or more of the mineral’s important features. Preparation Time: 30 min Background information: go to MINERAL FAMILIES document at the end for additional information on the mineral families. Activity time: 90 min Preparation Time: 30 min Before Activity: students should know characteristics of minerals so they can classify them. They also need to understand how to utilize guide books. After activity: posters should be presented to the whole class and then displayed Notes Questions for rock identifications are provided on the ROCK IDENTIFICATION following web site: USING FLOW http://www.rockhounds.com/rockshop/rockkey/index.html Each station has one card that asks a question. A student CHARTS Guiding Question: proceeds to the next station based on his/her answer. Each station is numbered and many of them have one distinct rock. Is the rock cycle If the station’s question requires the use of a tool or materials, truly cyclical? provide it at that particular station. Activity time: 30 min Preparation Time: 30 min Before Activity: The teacher needs to be sure students understand how to move around the room through the stations and the purpose of the activity. Earth/Environmental Science- Unit 3 DRAFT 45 A ROCK IS A ROCK Guiding Question: How are rocks classified? What are their differing characteristics? HISTORY ROCKS Guiding Question: What rock or mineral has had the greatest influence or impact on human society? After activity: students can write a story about their journey as a rock or they can discuss in whole group their findings Notes: This is a web based activity; SAS in School is a free resource for teachers in North Carolina. Find out if your school has already set up an account. If not, write to SAS: inschool@sasinschool.com to request an account. Activity time: 90 mins Preparation Time: 15 min Before Activity: The teacher should be proficient in this web activity and schedule computers for the class. The students should be aware of Internet etiquette and procedures for computer use. The SAS-in-School Curriculum Pathways Earth Science > Rocks, Minerals and Soil > Web lesson: “A Rock is a Rock” After activity: students will share their electronic reports and/or quiz Notes The teacher will need to be prepared to re-teach characteristics of writing or preferably partner up with the English teacher to ensure successful papers. More information on HISTORY ROCKS following this section of the document Before Activity: the teacher should have rubric for grading which is accessible to students After activity: Students should be able to present their findings to the class OREO COOKIE Notes: all the directions to this activity are on the website. You will need to emphasize that the cookie is eaten after the ACTIVITY Guiding Question: How can you simulate and relate plate movements? exercise is complete. Before Activity: purchase sandwich cookies and be sure students are acquainted with the terminology Goal *** Activities (*** - 90 min days) Teacher Notes LOCATING PATTERNS OF EARTHQUAKE AND VOLCANO DISTRIBUTION Notes: See Locating Patterns of Earthquates details at the end of this section of the document for more detailed information. Before Activity: Activity time: 90 min class period but students will need more time to write and revise essays Preparation Time: 5 min Activity time:30 min Preparation Time: 5 min After activity: discuss as whole group or in written descriptions of the experience and how modeling has helped to understand plate movement. Supplementary Notes Activity time: Preparation Time: After activity: Earth/Environmental Science- Unit 3 DRAFT 46 Guiding Question: What is the worldwide pattern of Earthquake and Volcano Distribution? EVIDENCE FOR PLATE TECTONICS Guiding Question: Notes SAS in School pathway: Earth Science > Plate Tectonics > InterActivity: “Evidence for Plate Tectonics” Earth Science > Plate Tectonics > Project: “Patterns of Fury” SAS-in-school is a free resource for teachers in North Carolina. Find out if your school has already set up an account. If not, write to SAS: inschool@sasinschool.com Before Activity: The teacher should use the website to practice the activity. The students should be aware of the theory of plate tectonics. DENSITY OF IGNEOUS ROCKS Guiding Question: After activity: A whole group discussion concerning what was learned through this activity Notes This activity is on the Spring Valley High School (New York) Earth Science website http://www.eram.k12.ny.us/education/components/docmgr/ default.php?sectiondetailid=17511&fileitem=2453&catfilter=5 67 Before Activity: The teacher needs to be sure students understand the difference between density and weight. The students may need a quick refresher on how do calculate. VIRTUAL EARTHQUAKE Guiding Question: After activity: Students will be able to calculate mineral percentages in a variety of igneous rocks using the Scheme for Igneous Rock Identification tables. Notes: The following website has step-by-step instruction and practice for determining the epicenter of an earthquake using real data. http://vcourseware5.calstatela.edu/VirtualEarthquake/V QuakeExecute.html The result of the triangulation at the end indicates whether or not the student has gained mastery. This would be a good activity to include in a computer lab packet. Activity time: 90 min Preparation Time: none but be sure to have computers available Activity time:45 mins Preparation Time: none but you will need to reserve computers Activity time: 50 min Preparation Time: none Before Activity: The teacher should become familiar with the possibilities presented in the website. Students need to review vocabulary. Earth/Environmental Science- Unit 3 DRAFT 47 LOCATING THE 1989 “WORLD SERIES EARTHQUAKE” EPICENTER Guiding Question: How do scientists calculate the epicenter of an earthquake? Guiding Question: After activity: students should submit a written explanation of their experience with the virtual earthquakes. Notes: This website gives students more practice finding an epicenter using real data. http://www.classzone.com/books/earth_science/terc/ content/ investigations/ es1003/es1003page02.cfm Activity time: 50 min Preparation Time: none Before Activity: Students should be aware of how a seismograph operates. After activity: Students should be able to relate their findings to the entire class or in written form. Activity time: Notes Before Activity: Preparation Time: After activity: Goal *** Activities (*** - 90 min days) Teacher Notes Supplementary Notes USING DISASTER TO TEACH SCIENCE Notes: Students will use a real-life eample of a natural-gas pipeline explosion to explore http://www.quantumsimulations.com/pdfs/ EBS_Article.pdf Activity time: 60 Guiding Question: Preparation Time: none Before Activity: The teacher needs to practice the web-based lesson before using it with the students. After activity: The students will be able to understand the math relationship that allows scientist to create models to forecast possible events. MINERAL FAMILIES AND FORMATION If you have a fairly substantial mineral collection and many of your students have already taken chemistry, this activity is a nice extension for understanding and developing an appreciation for how minerals form. Prepare for this multiple-day activity by organizing your mineral collection according to mineral families. Common mineral families include: the silicates, the carbonates, the oxides, the sulfides, the sulfates and the halides. There are numerous smaller mineral families. Materials: Mineral specimens from the families listed above grouped according to mineral family. Hand lenses. Rock and Mineral guidebooks (Simon and Schuster’s guidebook does a good job covering mineral families and environment of formation). Students may do their work in notebooks, journals or scrapbooks. Provide colored pencils and extra fine-tipped markers if they are available. Earth/Environmental Science- Unit 3 DRAFT 48 Directions: Each page or section in the student notebook will represent one type of mineral formation. The categories of mineral formation are as follows: 1 - ACCESSORY MINERAL IN BOTH IGNEOUS AND/OR METAMORPHIC ROCKS 2 - COMPONENT OF MAFIC AND ULTRAMAFIC IGNEOUS ROCKS 3 - COMPONENT OF FELSIC IGNEOUS ROCKS 4 - MINERALS THAT FORM UNDER METAMORPHIC CONDITIONS (contact or regional) 5 - FORMATION IN PEGMATITES 6 - FORMS IN HYDROTHERMAL MINERAL VEINS 7 - FORMED BY THE CHEMICAL ALTERATION OF ANOTHER MINERAL 8 - FORMS IN THE OXIDATION ZONE OF A MINERAL DEPOSIT 9 - FORMS DURING VOLCANIC OR ERUPTIVE EVENTS 10 - OCCURS IN PLACER DEPOSITS 11 - FORMS BY MAGMATIC SEGREGATION 12 - FORMS BY WEATHERING 13 - FORMED AS A PRECIPITATE BY EVAPORATION OF WATER 14 - FORMED AS A PRECIPITATE IN SEA WATER 15 - MINERAL PRECIPITATED AROUND HOT SPRINGS OR GEYSERS The student starts by selecting a mineral in the mineral family box from which he/she is working. Use on of the guidebooks or other resource to determine how the mineral is formed. This may also be referred to as “environment” or “occurrence.” Once this information is found, students will list the mineral on the specific mineral formation page. All or some of the following information can be recorded for each mineral: Name of mineral Chemical formula and its “English” translation i.e. Halite, NaCl, sodium chloride Geographic locations where mineral is found Uses for the mineral Associated minerals (commonly found along with mineral in question) The following are notes on mineral occurrence for selected minerals. Minerals are grouped according to family. Copy and laminate these notes if you do not have enough guidebooks. Included are the following families: silicates, carbonates, oxides and sulfides. Sulfates, halides and others are not represented in these notes. SILICATE FAMILY OF MINERALS (The “Rock-formers”) OLIVINE - Magnesium iron silicate Transparent variety called Forsterite (Mg2SiO4) is faceted as a gem. Peridot is gem-quality olivine not limited to the forsterite; it includes Fe2SiO4 A main constituent of mafic and ultramafic igneous rocks such as basalt, gabbro and peridotite ALMANDINE (Garnet Group) - Iron aluminum silicate Common mineral in medium-grade metamorphic environments Detrital mineral in sedimentary rocks due to its hardness and resistance to weathering Earth/Environmental Science- Unit 3 DRAFT 49 Used in medium-grade sandpaper; inexpensive gemstones. Crystals can be collected on NC-98 a few miles west of Wake Forest, NC. Formed when certain rocks undergo medium-grade metamorphism. Garnets often occur in river and beach sands. ZIRCON - Zirconium silicate Typical accessory mineral of acidic igneous rocks Found in beach sands; “heavy sand” Common accessory mineral in many igneous rocks – also in schists and gneisses. Rounded grains occur in placer deposits. SILLIMANITE - Aluminosilicate polymorph (Al2SiO5) Sensitive indicator of temp. and pressure at which host rock formed Widespread in high-temp. regional metamorphic rocks ANDALUSITE – Aluminosilicate polymorph (Al2SiO5) Used in high-temp. electrical insulators and acid-resistant ceramics; Occurs with pyrophyllite in Hillsborough. Very hard (7.5) Large crystals found in Andalucia, Spain. Typical of low-temperature metamorphic rocks that are rich in aluminum KYANITE - Aluminosilicate polymorph (Al2SiO5) Two hardnesses: 6-7 (across cleavage planes) 4-5 (along cleavage planes) Raw material for manufacture of high-temp. porcelain products and perfect electrical insulators. Found mainly in medium to high-grade regionally metamorphosed schists and gneisses. Diagnostic blue color. TOPAZ - Hydrous Aluminum silicate Largest and finest crystals come from Brazil and Siberia Deep golden-yellow variety is most highly regarded Found with pyrophyllite in Hillsborough Formation in pegmatites in felsic igneous rocks such as granites and rhyolites. STAUROLITE - Hydrous iron magnesium aluminum silicate Often found in alluvial sands due to insolubility. Very hard (7 – 7.5) Found as single and twinned crystals (cruciform) Forms in medium-grade metamorphic rocks. Also found in placer deposits. TITANITE - Calcium titanium silicate Large masses are worked as an ore of titanium Accessory mineral in “intermediate” igneous rocks such as diorite and syenite EPIDOTE - Calcium aluminum iron silicate A very common constituent of rocks This mineral filled what were once vesicles in basalts, which outcrop on New Hope Church Road near the NC 86 intersection. In regional and contact metamorphic rocks of mafic origin. Occurs in cavities in rocks such as gabbro, basalt and amphibolite. Earth/Environmental Science- Unit 3 DRAFT 50 BERYL - Beryllium aluminum silicate Characteristic of granitic rocks and pegmatites, where it sometimes occurs as enormous crystals Aquamarine (light blue), heliodor (yellow), morganite (pink) and emerald (green) are varieties of beryl. Spruce Pine, NC is a famous emerald locality. It is associated with quartz, spodumene, columbite, cassiterite, tantalite and other rare minerals. TOURMALINE - Complex borosilicate Elongated prismatic crystals that vary greatly in color. Striations parallel to the long axis resemble licorice. Can conduct electricity when strained (piezoelectric). Common accessory mineral in igneous and metamorphic rocks. AUGITE (pyroxene group) - Calcium magnesium iron aluminum silicate Common mineral in mafic and ultramafic igneous rocks such as gabbro, basalt and pyroxenite. Distribution very widespread. Cleavage angle of minerals in pyroxene group = 93 and 87 degrees. SPODUMENE (pyroxene group) - Lithium aluminum silicate Important industrial sourceof lithium. Valuable variety called “hiddenite” found in the western Piedmont of North Carolina. Found in lithium-bearing pegmatites associated with quartz, feldspars, lepidolite, beryl and tourmaline. ENSTATITE (pyroxene group) - Magnesium silicate Found in mafic and ultramafic plutonic and volcanic rocks, as well as in high-grade metamorphic rocks and in meteorites. ACTINOLITE (amphibole group) - Hydrous calcium magnesium iron silicate Found in an area of ultramafic rocks next to Falls Lake, north of Raleigh. Common in mafic metamorphic rocks. HORNBLENDE (amphibole group) - Complex silicate Green or black color with brown streak. Prismatic cleavage at 60 and 120 degrees. Distribution widespread and very common. An important constituent of igneous rocks and metamorphic rocks especially amphibolites. PREHNITE (phyllosilicates) - Hydrous calcium aluminum silicate Pale green; often found in “mammilary” aggregates. Found in fissures in igneous rocks and in some low-grade metamorphic rocks. PYROPHYLLITE (phyllosilicates) - Hydrous aluminum silicate Most often found as rosettes (radiating crystals). Used in cosmetics and paints; a dry lubricant and for heat and electrical insulation. Excellent specimens found in Hillsborough, NC. Pyrophyllite found in the Carolina Slate Belt formed from the chemical alteration of volcanic tuffs by geysers and hot springs. TALC (Phyllosilicates) - Hydrous magnesium silicate Very soft; Hardness=1 on Moh’s scale. Ingredient in paper and rubber; cosmetics and paints. Alteration product of magnesium silicates in ultramafic rocks Earth/Environmental Science- Unit 3 DRAFT 51 MUSCOVITE (Phyllosilicates) Often called “mica.” One of the most common minerals in rocks. Used in electrical or heat insulation. Garnet-mica schist outcrop on NC 98 between Durham and Wake Forest Formation in intrusive felsic igneous rocks. Enormous crystals found in pegmatites. Also found in some varieties of schists and gneisses BIOTITE (Phyllosilicates) - Hydrous potassium magnesium/iron aluminum silicate Often called “black mica.” Very large crystals (up to 70 square meters) found in pegmatites Component of felsic igneous rocks, gneisses and schists. VERMICULITE (Phyllosilicates) - Hydrated magnesium iron aluminum silicate When partially heated, becomes a very light thermal and accoustical insulating material. Used in the paper, paint and plastics industry. A hydrothermal alteration product of biotite especially at the contact between felsic intrusive rocks and ultramafic rocks KAOLINITE (Phyllosilicates, Clay Group) - Hydrous aluminum silicate Essential in the china industry and also used as a filler for paper and rubber, and in medicines and cosmetics. An alteration product of feldspar by chemical weathering and on a large scale by hydrothermal activity. CHRYSOCOLLA (Phyllosilicates) - Hydrous copper silicate Soft; light green to bluish. Decomposes in HCl forming a silica gel. Useful ore of copper; Found in Arizona and New Mexico. An important surface indicator, pinpointing the presence of disseminated deposits (porphyry copper ore) In the oxidation zone of copper deposits; associated with azurite, malachite and cuprite. ORTHOCLASE (Tectosilicate, potassium feldspar group) - Potassium aluminum silicate H=6 on Moh’s scale. Usually colorless, white, pale yellow, pink, blue or gray. Perfect 90 degree cleavage. Easily altered thru action of hot water rich in carbonic acid. Porcelain, ceramic glazes, electrical insulators, dental products. An essential component of many intrusive, plutonic rocks formed at medium-to-high temperatures and cooled slowly (granites. granodiorites, syenites, monzonites and pegmatities. MICROCLINE (Tectosilicate, Orthoclase Feldspar group) - Potassium aluminum silicate Blue-green variety called “amazonite.” Frequently found as twinned crystals. In granite pegmatites and metamorphic rocks (gneiss) formed at low to medium temperatures. ALBITE (Tectosilicate, Plagioclase Feldspar Group) - Sodium calcium aluminum silicate. Used in ceramics and refractories. Essential constituent of many felsic, plutonic igneous rocks. LABRADORITE (Tectosilicate, Plagioclase Feldspar Group) - Sodium calcium aluminum silicate. Sometimes strongly iridescent. Found in Labrador and Norway. Typical of eruptive rocks (basalts) and intrusive (gabbros). Earth/Environmental Science- Unit 3 DRAFT 52 QUARTZ (tectosilicate) CARBONATE FAMILY OF MINERALS MAGNESITE – magnesium carbonate Semi-hard (3-3.5), heavy (over 3.0) and fragile. Microcrystalline, porcelain-like masses with conchoidal fracture. Specimen is white. Effervesces with warm hydrochloric acid (HCl) Large deposits in the Coastal Ranges of California. Important ore of magnesium and its salts.Used in the manufacture of basic refractories capable of withstanding extremely high temp. and for special types of cement and powders used in the paper, rubber and pharmaceutical industries. Formed in talc schists and serpentinites (ultramafic rock) where carbonated water has reacted with serpentinite, which is rich in magnesium. SIDERITE – iron carbonate Rhombohedral crystals with curved, striated faces. Semi-hard, heavy and fragile. Dissolves in hot HCL with distinct effervescence. Large masses fond in veins in Lancaster County, PA and Roxbury, CT. An important iron mineral (48% Fe) because it is free of sulfur and phosphorus and sometimes rich in manganese. Very common in medium to low-temperature hydrothermal veins, associated with galena, fluorite, barite, sphalerite. RHODOCHROSITE – manganese carbonate. Semi-hard, heavy and fragile with a perfect rhombohedral cleavage. Vitreous to pearly luster. Pink coloration. Soluble in warm HCl. Found in many localities in Colorado. An ore of manganese when found in large masses. Used as a semi-precious gem. Found in medium-temp. hydrothermal veins associated with copper, silver and lead sulfides. CALCITE – calcium carbonate A limestone-forming mineral. Rhombohedral and “dogtoothed” varieties. Many colors. Often intergrown or twinned. Semi-hard, light with perfect rhombohedral cleavage. Soluble in cold, dilute HCl. Stalactites and stalagmites in limestone caves. A polymorph of calcite is aragonite. Calcareous rocks constitute 4% of the earth’s crust by weight and cover 40% of its surface. Golden-yellow crystals up to 1 meter in length found in Joplin, Missouri. Clear crystals were formally used to make polarizing prisms for petrological microscopes. Used in building (cement lime and ornamental stone), manufacture of fertilizers, and in the chemical industry. Formed in numerous geological situations. Most calcites have marine origins. May be formed by chemical precipitation through evaporation of solutions rich in calcium bicarbonate. Where lime-rich waters run through underground cave systems, calcite is deposited as stalactites and stalagmites. Also found in low-temp. hydrothermal veins associated with sulfides. ARAGONITE – calcium carbonate Small, elongated prismatic crystals often in radiating groups. Easy to see individual crystal. Many colors. Semi-hard, heavy and fragile. Found in deposits of hot springs (travertine). Fort Collins, CO, Arizona, New Mexico. Of interest to scientists and collectors. A highpressure polymorph of calcite, only stable in metamorphic rocks formed at high pressure and low temperature. Many marine organisms initially precipitate their shells as aragonite, which later convert to calcite. Earth/Environmental Science- Unit 3 DRAFT 53 DOLOMITE – calcium magnesium carbonate Colorless, white, pink or yellowish rhombohedral crystals. Semi-hard; vitreous and sometimes pearly luster. Dissolves slowly in cold HCl. Finest crystals come from Piedmont, Italy. In USA: Joplin, MO, Iowa, Vermont, Michigan and NJ. Formation as a hydrothermal mineral and also by the replacement of limestones by the action of magnesium-rich solutions. Frequently considered a rock-forming mineral. STRONTIANITE – strontium carbonate Needle-like (acicular) crystals, generally in bundles or sheaves. Colorless or white. Semihard, heavy and fragile with good prismatic cleavage. May be fluorescent blue under UV light. Soluble in dilute HCl. Economic deposits at Strontium Hills, CA. An ore for strontium, used in making fireworks (purplish-red flame). Used in sugar industry and for special types of glass. Typical of low-temp. hydrothermal veins associated with calcite, celestite and sulfides. Found in geodes. CERRUSITE – lead carbonate Colorless or white crystals with gray tints. Elongated and generally twinned. Semi-hard, very heavy and very fragile. Insoluble in HCl, however dissolves in nitric acid. Splendid crystals found in Dona Ana, NM, Phoenixville, PA and Leadville, CO. One of the ores for lead. Occurs in the oxidation zones of lead deposits, produced by the chem. alteration of galena (lead sulfide) through the actions of waters rich in carbonic acid. AZURITE – hydrous copper carbonate Semi-hard, heavy and fragile. Distinctive deep blue color. Often intergrown or grouped in radiating aggregates. Also earthy, granular or concretionary masses. Soluble in ammonia and effervesces in dilute acids. Pale-blue streak. The “copper district” of Arizona (SE corner, near Bisbee) A secondary mineral which occurs in the oxidized zones of copper deposits. Forms at lower temp. than malachite, which often replaces it as a pseudomorph through hydration. MALACHITE – hydrous copper carbonate Commonly occurs as green film on other copper minerals. Grape-like (botryoidal) masses. Distinctive bluish-green color. Light-green streak. Tintic district in Utah, and Bisbee, AZ. A very valuable, decorative stone used in polished slabs for tables, boxes and ornaments. In the past, it was crushed and used as an inorganic pigment. A copper ore. Found in oxidation zone of copper deposits. Sometimes occurs in large masses with a core of azurite. OXIDE FAMILY OF MINERALS ZINCITE- Zinc manganese oxide Crystals are rare. Usually massive, color ranges from orange to dark red. Semi-hard, very heavy, perfect cleavage. Translucent with subadamantine luster. Orange-yellow streak. Infusible. Soluble in hydrochloric acid. Found almost exclusively in Franklin and Sterling Hill (Sussex County, New Jersey), where it is associated with white or pink calcite, green willemite, grey tephorite and black franklinite. Crystals are sought after by mineralogists and collectors.. Formation in rocks of contact metamorphism where zinc is present. Earth/Environmental Science- Unit 3 DRAFT 54 FRANKLINITE-Zinc manganese iron oxide. Rare octahedral or dodecahedral crystals. Massive black aggregates with metallic appearance and reddish tints. Hard ( 5.5-6.5) , very heavy, fragile, no cleavage but good conchoidal fracture. Opaque with metallic luster. Reddish brown streak. Weakly magnetic. Infusible. Soluble in hydrochloric acid. Found in Sussex County, New Jersey along with zincite. In the past, used as extraction of zinc and manganese, which were then alloyed with iron. Formation in contact metasomatic conditions of zinc deposits in crystalline dolomite or limestone. SPINEL- Magnesium aluminum oxide Small, perfect octahedrons, frequently twined. Also common in aggregates of round grains. Colorless, white, pink, light blue, and black. Very hard (8). Heavy, poor cleavage. Transparent to nearly opaque with vitreous luster. White streak. Infusible and insoluble. Color is related to composition. Found in New York and New Jersey. Are semi-precious gemstones. Formation in contact metamorphosed limestones and in mafic to ultramafic igneous rocks; Also found in placer deposits. MAGNETITE- Iron Oxide Black, shiny, perfect octahedrons or dodecahedrons with striated faces. Iron black, compact and granular masses with bluish iridescence. Hard (5.6-6.5), very heavy, no cleavage. Opaque with metallic luster. Black streak. Strongly magnetic and sometimes acts as a natural magnet. Infusible. Soluble in very concentrated hydrochloric acid. It is the richest ore of iron. A very widespread accessory mineral in igneous rocks. Formation in high temperature mineral veins and as an accessory mineral in many mafic and ultramafic igneous rocks. Also found in banded iron formations. CHROMITE – Iron chromium oxide Rare, small black octahedral crystals, usually in compact granular masses. Hard and heavy with no cleavage. Dark brown streak. Weakly magnetic. Exclusively in mafic and ultramafic rocks. Found in South Africa, the Stillwater Complex, Montana, and in serpentines in Texas, Pennsylvania and California. The main ore for chromium. Steel alloys, stainless steel and leather tanning. Formation in mafic or utramafic igneous rocks; also occurs in placer deposits. CORUNDUM – aluminum oxide Hexagonal, barrel-shaped crystals with either tapered or stubby terminations. Variable in color. Very hard (H=9) Gem quality red corundum is called “ruby.” The blue gem quality corundum is called “sapphire.” An accessory mineral in silica-poor igneous rocks. Found in silica-poor and aluminum-rich metamorphic rocks. Sedimentary concentrations are found in alluvial or marine sands. HEMATITE – Iron oxide Most commonly found as granular masses, or sometimes found in an arrangement like the petals of a rose. Found in banded iron formations. Hard (5.5-6.5) and heavy (5.2). Streak is dark, cherry-red, which make it easy to distinguish from ilmenite and magnetite. Some of the largest deposits in the world found around Lake Superior. Hematite is the most important iron ore. Common mineral of many igneous rx., esp. lava. Common in pegmatites and hydrothermal veins, but NOT found in plutonic rocks (granite, for example). Much hematite Earth/Environmental Science- Unit 3 DRAFT 55 is formed under sedimentary conditions through diagenesis (the process by which sediments are changed to sedimentary rock )retaining the concretionary or oolitic forms. Hematite can also form as a sublimation product of volcanic exhalations. ILMENITE – Iron titanium oxide Dark mineral; hard, heavy, no cleavage. Weakly magnetic – becomes stronger when heated. Black to brownish-red streak. It is mined along with zircon at several locations in the Coastal Plain. A major ore of titanium. A common accessory mineral in plutonic igneous rocks. Also forms by magmatic segregation. Due to its resistance to weathering, large concentrations are found in marine sands. The black sand on North Carolina’s beaches is most likely ilmenite) RUTILE – Titanium oxide Elongated prismatic crystals, often striated. Sometimes very slender crystals occur as inclusions in other minerals. Hard and heavy with perfect cleavage. Nice specimens found in pyrophyllite in Graves Mountain, GA, and in hornblende-bearing rocks of Nelson County, VA A very common accessory mineral in a variety of igneous rocks such as granite. It is founding metamorphic rocks such as schist and gneiss. It can grow as needle-like crystals within quartz. CASSITERITE – Tin oxide Dark, stubby prismatic crystals, often twinned. Also found in black fibrous masses which look like wood. Hard (6-7) and very heavy (7) Occurs in pegmatitie veins of Oxford County, Maine and Custer County, South Dakota. Typical of hydrothermal veins around granite bodies. Also found in alluvial deposits. COLUMBITE – Iron manganese niobium tantalum oxide Prismatic, stubby or tabular crystals, often in heart-shaped twins, striated, black with metallic appearance and sometimes coated with iridescent film. Hard (6) and very heavy (up to 8) with distinct cleavage. Principal ore of niobium and tantalum. Found in granite-rich pegmatities rich in lithium silicates and phosphates, associated with spodumene and beryl URANINITE – Uranium oxide Occasionally found as black, cubic, octahedral or dodecahedral crystals. Usually in granular masses or aggregates. Physical properties are variable depending on the degree of radioactive decay of uranium into lead. Hard (5.5) and very heavy (up to 9.7) Shiny black streak. Highly radioactive. Deposits mined in the Colorado Plateau region of the Southwest (AZ, NM, CO, Utah) particularly on Navajo Indian lands. An Important source of uranium. Found in medium and high temperature hydrothermal veins. Also in clastic sedimentary deposits, which are sometimes gold-bearing. BAUXITE – Hydrous aluminum oxides Bauxite is a general term for a rock composed of hydrated aluminum oxides rarely found in distinct separate crystals. Very soft (1-3), light and fragile with earthy fracture. Found in Massachusetts and Arkansas. The main ore of aluminum. Also used in the synthetic production of corundum. Formed by decay through weathering, under tropical conditions, of rocks containing silicates of aluminum; heavy tropical rain leaches the silica and aluminum hydroxides remain. Earth/Environmental Science- Unit 3 DRAFT 56 LIMONITE – Hydrated iron oxides Limonite is a field term used to describe a rock made up of a mixture of mainly amorphous mineral-like substances. The term is also applied generally to any iron hydroxide which cannot be identified compositionally without elaborate tests. It is widespread and occurs as a secondary mineral in weathering zones but large masses develop as precipitates in both marine and fresh water and in bogs. SULFIDE FAMILY OF MINERALS BORNITE – copper iron sulfide Compact granular masses of a reddish-brown color, which quickly tarnishes on the surface showing an iridescent purple and blue film (hence the nickname –“peacock ore”). Soft (3) and heavy (5.7) Found at Butte, Montana and Bristol, CT. One of the most important industrial copper ores. Found in the oxidation zones of copper deposits; associated with malachite. Formation in hydrothermal veins and from magmatic segregation. SPHALERITE – zinc sulfide Variety of colors; aggregates of distorted crystals; octahedral twins with striated faces common. Semi-hard (3.5-4) and heavy (4) Streak pale yellow or reddish. Soluble in HCl. The world’s main deposits are in the Tri-State mining district (Missouri, Kansas, Oklahoma) The main ore for zinc (one of the components of brass and other alloys) Found in hydrothermal veins; associated with galena, chalcopyrite, fluorite and barite. CHALCOPYRITE – copper iron sulfide Generally in compact or microgranular masses; dark or brassy yellow in color, often with iridescent film. Greenish-black streak; softer than pyrite. Found in Butte, Montana; Jerome, AZ, and Bingham, Utah. One of the most important copper ores (80% of world’s copper derived from this mineral) Yields the by-products – gold and silver. Formation in hydrothermal veins, in porphyry copper deposits, as veins in diorites and in thermally metamorphosed rocks. PYRRHOTITE – iron sulfide Crystals usually tabular with faces striated horizontally. Commonly in yellow-brown massive, granular aggregates. Bright, metallic luster. Distinguished from pyrite by its ferromagnetism. Fine crystals occur in Standish, Maine and Brewster, NY. GALENA – lead sulfide Cubic, lead-gray crystals, metallic luster. Soft (2.5-2.8) and very heavy. Perfect 3directional cleavage (90 degrees). Soluble when heated in HCl, giving off a hydrogen sulfide odor (like that of rotten eggs). Found in abundance in the Tri-State mining district. The main ore of lead. Typical hydrothermal vein mineral of medium temperature deposits. CINNABAR – mercury sulfide Normally occurs in scarlet microcrysalline earthy masses. Soft (2-2.5) and very heavy (8.1) Volatile if heated above 580 deg. C (1076 deg. F) Occurs in very low temp. hydrothermal deposits. Small, well-formed crystals found in Pike County, Arkansas. The most important ore of mercury. It was used in the past as the mineral pigment known as vermilion. Formation in association with realgar and pyrite. It occurs in hot springs and around recent Earth/Environmental Science- Unit 3 DRAFT 57 volcanic vents. It has also been found in placer deposits from the erosion of mercury-bearing rock. PYRITE – iron sulfide Striated cubic or octahedral crystals, sometimes occuring as “iron cross” twins. Hard (6-6.5) and heavy (5) with poor cleavage. (the cubic shape of Pyrite is the result of the crystal itself. If broken, it would fracture.) Produces sparks when hit with a hammer. Splendid crystals occur all over the world. Some of the largest pyrite crystals in the world found imbedded in the pyrophyllite of Glendon, NC (Moore County) Used in the manufacture of sulfuric acid. Formation in a very wide-range of geological situations. Associated with sphalerite and galena in hydrothermal veins. It can be an accessory mineral in igneous rocks. It can be found in slates and dark shales where it often replaces fossils and forms nodules. ARSENOPYRITE – iron arsenic sulfide Elongated, striated, prismatic crystals; twins common. Also granular masses of silvery or whitish-gray color with pink tints. Hard (5.5-6) and heavy (6). Produces sparks when hit with a hammer and gives off a strong garlic odor. Roxbury, CT and Leadville, CO. The principal ore of arsenic with tin, gold, silver and cobalt as by-products. Formation in veins of hydrothermal origin often with gold, silver, tungsten and tin. MOLYBDENITE – molybdenum sulfide Bladed, foliated or finely interwoven masses common; bluish-gray color. Very soft (1-1.5) and heavy (4.7); greasy feel; metallic luster; bright-blue streak. Leaves a grayish-green mark on paper. Found in Edison, NJ and near Medoc Mountain State Park in eastern NC. The main ore of molybdenum, a metal used in many special alloys. Also used as a dry lubricant resistant to high temperatures. Found in granites as an accessory mineral and in quartz and pegmatite veins. REALGAR - arsenic sulfide Normally found as compact aggregates and scarlet films. Soft (1.5-2) and fairly heavy (3.5). Streak is red to orange. Fuses easily, giving off arsenic fumes with a garlic smell. If exposed to light and air, it loses its scarlet color and turns into powdered orpiment. Used in fireworks (bright white). A mineral deposited in hot springs and low temperature hydrothermal veins, almost always associated with orpiment and antimony, silver, lead and tin minerals. ORPIMENT – arsenic sulfide Found in crusts or bladed masses; soft and fairly heavy. Color and streak is yellow. Fuses easily giving off arsenic fumes with a strong garlic smell. Found in Nevada. An ore of arsenic; used in the tanning of hides to remove hair. Deposited in hot springs; associated with realgar and sometimes cinnabar. Earth/Environmental Science- Unit 3 DRAFT 58 HISTORY ROCKS Thomas Rhindress - Croton-Harmon High School ESSAY THOUGHT QUESTION - What rock or mineral has had the greatest influence or impact on human society? Support your answer. For example, oil and water are not minerals or rocks, but would be fantastic answers if they were. If you need ideas for a choice, look at the rock and minerals charts in the Earth and Environmental Reference Tables. Do NOT limit yourself to just those choices... 1. Write-up your answer to this question as a 1 to 2 page essay. 2. Type your essay - 12 pt font, double spaced, 1 inch margins. 3. Include a brief description of the mineral or rock: General physical and chemical traits, etc. Where it is found and/or how it is formed How it is obtained - mined or extracted What is it used for? How has its use been important to human society? How would society be different without it? 4. Cite any sources - Use Google to search for information on your choice. Then include the web address as your citation. Put these on the back of your essay, or on a separate stapled page. They do NOT count towards your length. 5. You may include ONE small picture, diagram, or illustration. Make sure it refers to something you are writing about. Cite its source, too. If you include more pictures, attach them on a separate sheet. 6. Essays will be graded on 1) scientific content and accuracy, 2) historical content, and 3) grammar, spelling, organization, and originality. Earth/Environmental Science- Unit 3 DRAFT 59 EVALUATE: Sample Assessment Questions 1. What type of mineral grain would be abundant in a soil underlain by granite? A) Quartz B) Mica C) Olivine D) Feldspar 2. One of the most common minerals in beach sand is quartz. The following properties of quartz account for its abundance except A) Quartz crystals fracture rather than exhibit cleavage B) Quartz is unreactive chemically C) Quartz is a hard mineral D) Quartz, over time, physically weathers into smaller and smaller pieces. 3. The data table below shows the composition of six common rock forming minerals. Mineral Mica Olivine Orthoclase Plagioclase Compostion KAl2Si2O16 (Mg,Fe)2SiO4 KAlSi3O8 NaAlSi3O8 CaAl2Si2O8 (Mg,Fe)2 SiO6 (Ca,Mg)2SiO6 SiO2 Pyroxene Quartz 4. What mineral family do the 6 common minerals belong? A) Silicates B) Oxides C) Carbonates D) Sulfides 5. How many different types of elements are found in the 6 common minerals? A) 8 B)6 C) 4 D) 12 6. Which of the following substances is considered a mineral? A) seawater B) salt C) cast iron D) vegetation 7. Large crystals with well-formed crystal faces tend to form when_______. A) molten rock cools quickly B) rocks undergo melting C) minerals have to space to grow, such as in open cavities D) volcanoes erupt explosively 8. Iron and magnesium ions are similar in size and both have a “+ 2” positive charge. Therefore, we would expect iron and magnesium to ____. A) bond easily Earth/Environmental Science- Unit 3 DRAFT 60 B) share electrons C) magnetically repel each other D) substitute for each other in a mineral’s chemical formula 9. Examples of substances that have exactly the same chemical formula but different crystal structures are A) B) C) D) orthoclase and plagioclase feldspar diamond and graphite mica and talc quartz and fluorite 10. Most common rock-forming minerals are________. A) carbonates B) oxides C) silicates D) sulfides 11. The two most common elements in the Earth’s crust are: A) calcium and carbon B) chlorine and sodium C) iron and sulfur D) silicon and oxygen 12. The mineral property “cleavage” refers to _____. A) B) C) D) The development of crystal faces during mineral growth The splitting of a mineral along planar surfaces The development of irregular fracture in a mineral The density of a mineral 13. When a mineral does not break evenly is exhibits _____ (terms that describe this feature include conchoidal, fibrous and splintery) A) B) C) D) Habit Frcture Cleavage Luster 14. When igneous rock is formed, the size of the rock crystals or grains is primarily dependent on a) cooling rate of the magma b) volume of the magma c) density of the magma d) amount of impurities in the magma 15. Which would most likely occur during the formation of igneous rock? Earth/Environmental Science- Unit 3 DRAFT 61 a) solidification of molten materials b) compression and cementation of sediments c) recrystallization of unmelted material d) evaporation and precipitation of sediments 16. Rock A and Rock B are igneous rocks with identical mineral composition. Rock A has large visible crystals and Rock B has no visible crystals. What can be inferred about Rock A? a) it cooled beneath the earth’s surface, more slowly than Rock B b) it cooled at the earth’s surface, more slowly than Rock B c) it cooled at the earth’s surface, more quickly than Rock B d) it cooled beneath the earth’s surface, more quickly than Rock B 17. Which feature is characteristic of clastic sedimentary rocks? a) layering b) intergrown crystals c) foliation d) glassy texture 18. Which sequence of events occurs in the formation of a sedimentary rock? a) source material eroded > sediments deposited > sediments compacted and cemented b) sediments compacted and cemented > sediments deposited > source material eroded c) sediments deposited > sediments compacted and cemented > source material eroded d) source material eroded > sediments compacted and cemented > sediments deposited 19. Sedimentary rocks of organic origin would most likely be formed from a) shells of marine animals b) materials deposited by glaciers c) sediments eroded from running water d) particles removed from the atmosphere by precipitation 20. What is the main difference between metamorphic rocks and most other rocks a) many metamorphic rocks exhibit banding and distortion of structure b) many metamorphic rocks contain only one mineral c) many metamorphic rocks have an organic composition d) many metamorphic rocks contain a high amount of silicon-oxygen tetrahedral 21. Which characteristics are most useful for identifying the conditions under which a metamorphic rock was formed? a) composition and structure b) color and luster c) shape and mass d) hardness and size Earth/Environmental Science- Unit 3 DRAFT 62 22. What characteristic provides the best evidence about the environment in which a rock formed? a) the texture of the rock b) the color of the rock c) the size of the rock d) the thickness of the rock 23. Which statement about the formation of a rock is best supported by geologic evidence? a) sediment must be compacted and cemented before it can change to a sedimentary rock b) magma must be weathered before it can change to a metamorphic rock c) sedimentary rock must melt before it can change to a metamorphic rock d) metamorphic rock must melt before it can change to a sedimentary rock? 24. Which of the following statements about transportation of sediment is false? A) Smaller particles settle faster than larger particles. B) As a current slows, the largest particles start to settle C) Faster currents carry larger particles than slower currents D) Rivers and ocean currents move much more material than do air currents 25. Fossils are usually found in A) igneous B) sedimentary C) metamorphic D) lava rock 26. Which set of Earth processes are thought to be most closely related to each other because they normally occur in the same zones? A) mountain building, earthquakes and volcanic activity B) earthquakes, shallow-water fossil formation, and shifting magnetic poles C) volcanic activity, rock weathering, and deposition of sediments D) mountain building, shallow-water fossil formation and rock weathering 27. The occurrence of high temperature ocean floors at mid-ocean ridge centers is an indication of A) existence of rising mantle convection currents B) destruction of ocean crust C) destruction of continental crust D) existence of ancestral mountains 28. Mid-ocean ridges such as the Mid-Atlantic Ridge and the East pacific Rise are best described as A) sections of the ocean floor that contain the youngest oceanic crust B) mountains containing folded sedimentary rocks C) mountains containing fossils of present-day marine life D) sections of the ocean floor that are the remains of a submerged continent Earth/Environmental Science- Unit 3 DRAFT 63 29. Which observed feature would provide the best evidence of crustal stability? A) horizontal sedimentary layers B) folded, faulted and tilted rock strata C) marine fossils at elevations high above sea level D) changed benchmark elevations 30. Along the ocean floor near mid-ocean ridges, hot springs may be found. These hot springs provide evidence that A) convections currents exist in the asthenosphere 31. Which inference is best supported by the Moon’s apparent absence of continental drift? A) The Moon lacks convection currents in its mantle 32. Molten rock below the Earth’s surface is called A) magma B) lava C) mantle D) gabbro 33. Which of the following types of lava flows downhill the slowest? A) rhyolite B) basalt C) andesite D) they all flow at about the same speed? 34. Which of the following statements about lava is false? A) High temperature lavas are more viscous than low temperature lavas B) The viscosity of a lava increases as the silica content increases C) The more gas a lava contains, the more violent the eruption D) all of the above statements are true? 35. Which of the following rocks is formed from volcanic ash? A) tuff B) pahoehoe C) breccia D) basalt 36. The general term for solidified fragments of volcanic material that are ejected into the air and cool rapidly is A) pyroclast B) granite C) obsidian D) basalt 37. The slope of a cinder cone is determined by which of the following? A) the maximum angle at which pyroclastic debris remains stable B) the viscosity of the lava erupting from the cone Earth/Environmental Science- Unit 3 DRAFT 64 C) the force at which the eruption occurred D) the slope of the topography surrounding the volcano 38. Which of the following types of volcanic features does not result from an eruption from a central vent A) flood basalts B) cinder cones C) stratovolcano D) volcanic dome 39. Which of the following gases is the chief constituent of volcanic gases? A) water vapor B) carbon dioxide C) sulfur dioxide D) oxygen 40.In which of the plate tectonic settings do most of the world’s active volcanoes occur? A) convergent plate boundaries B) divergent plate boundaries C) transform plate boundaries D) hot spots in the middle of plates 41. Which set of Earth processes are thought to be most closely related to each other because they normally occur in the same zones? A) mountain building, earthquakes and volcanic activity B) earthquakes, shallow-water fossil formation, and shifting magnetic poles C) volcanic activity, rock weathering, and deposition of sediments D) mountain building, shallow-water fossil formation and rock weathering 42. The occurrence of high temperature ocean floors at mid-ocean ridge centers is an indication of A) existence of rising mantle convection currents B) destruction of ocean crust C) destruction of continental crust D) existence of ancestral mountains 43. Mid-ocean ridges such as the Mid-Atlantic Ridge and the East pacific Rise are best described as A) sections of the ocean floor that contain the youngest oceanic crust B) mountains containing folded sedimentary rocks C) mountains containing fossils of present-day marine life D) sections of the ocean floor that are the remains of a submerged continent 44. Which observed feature would provide the best evidence of crustal stability? A) horizontal sedimentary layers B) folded, faulted and tilted rock strata C) marine fossils at elevations high above sea level Earth/Environmental Science- Unit 3 DRAFT 65 D) changed benchmark elevations 45. Mineral-rich superheated waters due to proximity to magma form ores through this process A) hydrothermal activity B) 46. Which pair of metals are the ones used the most on Earth? A) iron and aluminum B) zinc and copper C) silver and gold D) brass and lead 47. What is the difference between a reserve and a resource? 48. How is the exploration of minerals supported by geological maps? A) They show the type of bedrock that lies beneath the surface B) They show exact spots where drilling should take place C) They show how deep the mineral deposits are located D) They show the mineral composition of the bedrock 49. What is the advantage of using aluminum for beverage containers? A) Making aluminum from recycled products is not expensive B) Of all the metals, aluminum is the only one that can be recycled C) Less energy is needed in the manufacturing of aluminum compared to steel D) It does not require much energy to turn aluminum ore into aluminum 50. Which of the following types of bedrock would make the best oil reservoir? A) sandstone B) granite C) shale D) salt 51. Which fossil fuel produces the least amount of carbon dioxide per unit of energy? A) oil B) natural gas C) coal 52. What are the prerequisites for oil traps to contain oil? 53. Which of the following geological conditions is least likely to create an oil trap? A) s syncline B) a fault C) an anticline D) an igneous pluton 54. PROVIDE DIAGRAM – UNDERSTANDING EARTH TEST BANK P. 239 Below is a cross-section of an oil trap. J, K and L represent three layers of fluids, each layer having a slightly different density from the other. (DIAGRAM) 55. The oil trap depicted above is a(n) A) anticlinal trap Earth/Environmental Science- Unit 3 DRAFT 66 B) salt dome trap C) stratigraphic trap D) fault trap 56. If the pore spaces in layer B contain oil, what will the pore spaces in layer C likely contain? A) water B) natural gas C) coal D) air 57. Which type of sedimentary environment would most likely produce coal? A) swampy environment B) shallow ocean environment C) volcanic environment D) desert environment 58. Which of the following problems is not directly associated with the burning of coal? A) global warming B) ash with metal impurities C) carbon dioxide D) acid rain RESOURCES for part 1 - Minerals The Earth Revealed http://www.learner.org/resources/series78.html A video instructional series on geology for college and high school classrooms and adult learners; 26 half-hour video programs and coordinated books This series shows the physical processes and human activities that shape our planet. From earthquakes and volcanoes to the creation of sea-floor crusts and shifting river courses, Earth Revealed offers stunning visuals that explain plate tectonics and other geologic concepts and principles. Follow geologists in the field as they explore the primal forces of the Earth. This series can also be used as a resource for teacher professional development. Mineral Links http://www.minsocam.org/MSA/Research_Links.html#mineral_locality Dana Classification of Minerals http://webmineral.com/danaclass.shtml Virtual Atlas of Opaque and Ore Minerals http://www.smenet.org/opaque-ore/ Mineral Spectroscopy – California Institute of Technology http://www.smenet.org/opaque-ore/ Minerals of Western North Carolina http://www.mcrocks.com/index.html USGS Mineral Resources web site http://minerals.usgs.gov/ Mineral Gallery http://mineral.galleries.com/ Earth/Environmental Science- Unit 3 DRAFT 67 USGS publication – The Life Cycle of a Mineral Deposit – a free online guide for teachers http://pubs.usgs.gov/gip/2005/17/ RESOURCES for part 2 – ROCKS The Earth Revealed http://www.learner.org/resources/series78.html A video instructional series on geology for college and high school classrooms and adult learners; 26 half-hour video programs and coordinated books This series shows the physical processes and human activities that shape our planet. From earthquakes and volcanoes to the creation of sea-floor crusts and shifting river courses, Earth Revealed offers stunning visuals that explain plate tectonics and other geologic concepts and principles. Follow geologists in the field as they explore the primal forces of the Earth. This series can also be used as a resource for teacher professional development. EarthNet Rock Identification http://earthnet-geonet.ca/activities/rock_id_e.php Rock, Minerals and Soils http://www.seafriends.org.nz/enviro/soil/rocktbl.htm About Geology: Rock Identification Tables http://geology.about.com/library/bl/blrockident_tables.htm Igneous Rocks http://www.gpc.edu/~pgore/geology/geo101/igneous.htm Earth/Environmental Science- Unit 3 DRAFT 68 Earth/Environmental Science- Unit 3 DRAFT 69 RESOURCES for part 3 – earthquakes The Earth Revealed http://www.learner.org/resources/series78.html A video instructional series on geology for college and high school classrooms and adult learners; 26 half-hour video programs and coordinated books This series shows the physical processes and human activities that shape our planet. From earthquakes and volcanoes to the creation of sea-floor crusts and shifting river courses, Earth Revealed offers stunning visuals that explain plate tectonics and other geologic concepts and principles. Follow geologists in the field as they explore the primal forces of the Earth. This series can also be used as a resource for teacher professional development. Mineral Links http://www.minsocam.org/MSA/Research_Links.html#mineral_locality Dana Classification of Minerals http://webmineral.com/danaclass.shtml Virtual Atlas of Opaque and Ore Minerals http://www.smenet.org/opaque-ore/ Mineral Spectroscopy – California Institute of Technology http://www.smenet.org/opaque-ore/ Earth/Environmental Science- Unit 3 DRAFT 70 Minerals of Western North Carolina http://www.mcrocks.com/index.html USGS Mineral Resources web site http://minerals.usgs.gov/ Mineral Gallery http://mineral.galleries.com/ USGS publication – The Life Cycle of a Mineral Deposit – a free online guide for teachers http://pubs.usgs.gov/gip/2005/17/ RESOURCES for part 4 – plate tectonics The Earth Revealed http://www.learner.org/resources/series78.html A video instructional series on geology for college and high school classrooms and adult learners; 26 half-hour video programs and coordinated books This series shows the physical processes and human activities that shape our planet. From earthquakes and volcanoes to the creation of sea-floor crusts and shifting river courses, Earth Revealed offers stunning visuals that explain plate tectonics and other geologic concepts and principles. Follow geologists in the field as they explore the primal forces of the Earth. This series can also be used as a resource for teacher professional development. http://www.minerals.si.edu/tdpmap/ This Dynamic Planet (USGS map) This is a very comprehensive plate tectonics map that shows earthquakes and volcanoes, as well as plate movement (direction and distance in cm per year). You can order these maps through USGS. Recommended: A class set consisting of one map for every four students) American Geological Institute http://www.agiweb.org/ Ring of Fire lesson plan http://www.nationalgeographic.com/xpeditions/lessons/15/g912/ring.html Plate tectonics animation http://www.ucmp.berkeley.edu/geology/anim1.html USGS – Understanding Plate Motions http://pubs.usgs.gov/publications/text/understanding.html Discover our Earth - Plate Tectonics http://www.discoverourearth.org/student/tectonics/index.html A Model of Sea Floor Spreading – Teacher Guide http://www.ucmp.berkeley.edu/fosrec/Metzger3.html Plate Tectonics with an orange peel http://www.coaleducation.org/lessons/wim/10.htm USGS Plate tectonics earthquakes – volcanoes http://vulcan.wr.usgs.gov/Glossary/PlateTectonics/description_plate_tectonics.html Earthquakes, volcanoes and plate tectonics – slide show http://www.science.sjsu.edu/scied/255/PPT02/PlateTectonics.htm Earth/Environmental Science- Unit 3 DRAFT 71 Science links: earthquakes, volcanoes and plate tectonics http://www.sciencespot.net/Pages/kdzethsci2.html Interdisciplinary learning module – earthquakes, volcanoes and plate tectonics http://www.neiu.edu/~llsander/earthquakes.html Internet School library media center http://falcon.jmu.edu/~ramseyil/plate.htm tectonic applications http://edc.usgs.gov/Tectonic/ Volcano Slide Show http://volcano.und.nodak.edu/vwdocs/EOSslides/francis_slides.html USGS Volcano Hazards Program http://volcanoes.usgs.gov/About/Where/WhereWeWork.html Cascade Volcano Observatory http://vulcan.wr.usgs.gov/ USGS Yellowstone Volcano Observatory http://volcanoes.usgs.gov/yvo/ USGS – Volcano Hazards Program: Long Valley Observatory http://lvo.wr.usgs.gov/index.html#glance Volcanic landforms, volcanoes and plate tectonics http://www.tulane.edu/~sanelson/geol204/volclandforms.htm The Physical Environment: Tectonics and Landforms http://www.uwsp.edu/geo/faculty/ritter/geog101/textbook/tectonics_landforms/title_page.ht ml The Physical Environment: Volcanics and Landforms http://www.uwsp.edu/geo/faculty/ritter/geog101/textbook/volcanic_landforms/title_page.htm l Dive and Discover’s Deeper Discovery Hydrothermal vents interactive http://www.divediscover.whoi.edu/vents/vent-infomod.html# WEB RESOURCES for part 5 – economic development of the earth NASA Earth Science web site http://science.hq.nasa.gov/earth-sun/science/earth.html USGS publication – The Life Cycle of a Mineral Deposit – a free online guide for teachers http://pubs.usgs.gov/gip/2005/17/ Minerals in the average home (State of Nevada – Division of Minerals) http://minerals.state.nv.us/forms/educ/DidYouKnowHome_LH.pdf Minerals needed “to maintain our standard of living” (State of Nevada – Division of Minerals) http://minerals.state.nv.us/forms/educ/Inoneday_LH.pdf Fourth of July Fireworks (State of Nevada – Division of Minerals) http://minerals.state.nv.us/forms/educ/FourthOfJuly.pdf Ecological Footprint quiz http://www.myfootprint.org/ Earth/Environmental Science- Unit 3 DRAFT 72