Work instruction 12 Safe working with animal
advertisement

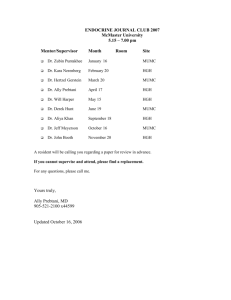
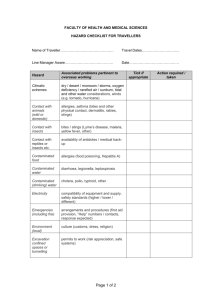
Biosafety Unit, CRISP, FHML, MUMC+ Work instruction 12: Receiving, sending and safe working with animal tissue and body fluids 1. Introduction This work instruction is based on the Decree Animal By Products (VO 1069/2009), the-execution decree 142/2011 and a number of articles from the Health and Safety decree. Animal by products are: animal cells, tissues, organs and other parts of animals that are not meant for human consumption, as well as body fluids like blood. Depending on their possible risks these products are categorized in Category 1 to 3, in which 1 is the highest risk. (see Documentation) The MUMC+ has a permit from the Ministry of VWS for the use of these products. The supervising authority is the Dutch Food and Commodity Authority (Nederlandse Voedsel en Warenautoriteit (NVWA)). Next to this instruction there is a document with background information about the risks of working with animal material, the Decree Animal by products and the relevant parts from the Health and Safety decree. The necessary forms are included under Forms 1 Receiving material from cat. 1 and 2 1.1 From the Netherlands and EU countries 1 2 3 Archive within the department the form filled in and signed by the sender and transporter, simplified accompanying commercial document and the delivery receipt for a minimum of 2 years. If you go and get the animal material yourself, make a trade document in duplicate and have one copy signed by the supplier and leave it with the supplier. The other signed copy must be taken back for archiving in your own department. This must be kept for 2 years. Store the material in a cool place or freezer that is provided with indication Cat.1 in the framework of the Decree Animal By Products (sticker obtainable from the Biosafety Officer (BSO). Keep a clear record of the archive for third party’s (f.i. the Inspection) consisting of: date of reception of the material, type of material, category and amount. 1.2 From remaining countries 1 2 When receiving material from non EU states like for instance Switserland, USA etc. ask for a continuing import release form at the NVWA, this will be valid for 2 years for the material concerned and for the institute doing the sending. See: Approval form import release animal tissue The original import release can be sent to the transprter, who must hand over the document to Veterinarian Institute. Keep one copy for your own archive. Version 02, jan. 2012 Biosafety Unit, CRISP, FHML, MUMC+ 3 4 Archive the delivery bill within the department and keep for a minimal of 2 years Store the material in a cool place or freezer, tagged Cat. 1 in the framework of the Decree Animal By Products and keep a clear administration, which is also clear for a third party (f.i. the Inspection); consisting of at least: date of receipt of the material, type of material, category and amount. 2 Activities 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 Make a Risk Assessment before starting the activities into the area of the specific animal material. This goes in particular for infected small laboratory animals and material from big laboratory animals, like goats, sheep, dogs and pigs. If needed ask the safety and Health Officer (Armico),the prevention employee, or the BSO for advice. Make a work instruction based on the Risk Assessment for the activities involved citing this Work Instruction and the work instruction for laboratories Wear a closed lab coat that remains in the lab. If needed wear nitrile gloves (if your skin is not intact). If aerosol forming is to be expected work in a microbiological safety cabinet (MVK). If this is not possible, use a breathing mask with FFP2L-filter If splashes are to be expected, work in a MVK or fume hood or use safety glasses. The pipette must be left to drain along the inner surface of the glass ware of the tube, to prevent aerosol formation. Always centrifugate in closed tubes. If an open centrifuge tube is needed, use a closed bucket and open it in a safety cabinet. In case of damage of the tubes, open the rotor in a safety cabinet and after cleaning disinfect with an appropriate disinfectant (see Risk Assessment and Instruction on disinfection of laboratories ) When suction is being done under vacuum make sure to use a reception bottle with a chlorine tablet (2 tablets Stafilex for a 2 liter bottle). Empty the bottle after at least 10 minutes ramp-up time. (See Instruction on disinfection of laboratories ) After finishing the activities clean the work surface with water and soap and disinfect with a suitable disinfectant. Soak up spillage with a tissue (wear nitrile gloves) and dispose off in the waste barrel for micro biological contaminated waste. After that cover the space with a 0.1% chlorine solution from a organic chloride connection (see Instruction on disinfection of laboratories) and leave this to soak for 5 minutes. Soak up everything with a tissue and dispose also in the waste barrel for biological contaminated material. After that clean the contaminated surface with water and soap. When there is an incident with breakage of tubes or other glassware use tweezers to pick up and dispose of the shards in the waste barrel for contaminated biological waste. Have the contaminated materials that can be re-used autoclaved (CGSA, UM). Biological waste must be disposed of in the waste barrels for specific hospital waste (blue barrels with yellow lid). Transport of biological waste within the MUMC+ (the institute that got permission) must be done in a closed, leak proof, unbreakable container, that has been disinfected on the outside with a suitable disinfectant. Version 02, jan. 2012 Biosafety Unit, CRISP, FHML, MUMC+ 3. Sending 4.1 within the Netherlands and the EU 1 2 3 4 5 Provide the package with cat. 1 or 2 material with two copies (keep the original yourself) of the accompanying Commercial Document, signed by you and the transporter. One copy of the Commercial Document is meant for the transporter and the other one for the recipient. Fill in the NVWA approval number for working with animal by products at the subject “sender”. For MUMC+ this number is: 35928 When sending a package to a Dutch Institute it is obligatory to fill in the approval number from that institute. Sign the trade document with a blue pen. The tissue has to be packed in triple containment and labeled with a label UN 3373, Biological Substance B, For Research and Diagnostics Only. (Etiket dierlijk materiaal) Archive one copy of the trade document within your department for 2 years. The trade document consists of the date that the material was collected/sent, and name and address of transporter and recipient. 4.2 to non EU countries 1 2 Make inquiries with the recipient about the rules for sending the material concerned to the country concerned. Archive one copy of the necessary forms. 4. Storage 1 2 3 Always keep animal material at a maximum of 40C and keep it separate from inflammable or explosive matter (guideline storage dangerous matter, PGS). When keeping it for longer freeze at -200 or at -800 (if this is not alien to the desired activity of the biological material). Label and register the stored material in such a way that it is clear for any third party (f.i. Inspection). Label the fridge or freezer, in which the material is stored with the sticker Cat. 1 (obtainable from BSO). 5. Waste Version 02, jan. 2012 Biosafety Unit, CRISP, FHML, MUMC+ 1 2 3 Dispose of the fixed material like possible contaminated waste (green or blue container, yellow lid). Disinfect the outside after closing the container with an appropriate disinfectant. Leave the container in the laboratory until it gets picked up (UM) or take it to the nearest waste collection area. Disposal of dead animals is done by Rendac through CPV. Fluid waste must be autoclaved by CGSA (UM) or disinfect with chloride tablets (see Instruction Disinfection in Laboratories and laboratory animal spaces. 6. Accidents and Incidents 1 2 3 4 5 6 7 8 Disinfect the skin of contaminated people with chloride hexidine in 70% alcohol or with iodine tincture. In case of a prick incident, that exposes you to animal material, firstly block the finger and rinse under water tap then disinfect with betadine iodine, chloride hexidine or 70% ethanol. After that go to the first aid (azM). In case of a cutting incident with considerable blood loss: quell the bleeding and go to first aid (azM). Report prick- and cut incidents, that expose you to animal material through the form Ongevallen on the UM site Reporting incidents and accidents through a VIM report (azM). In case of an injury caused by working with material from big laboratory animals one must always got to the first aid of the azM, this because of a possible tetanus injection. In case of an injury, call the first aid officer (BHV) at 1333 (UM) or 1000 (azM), so first aid can be given quickly and adequately. Rinse contaminated eyes for 10 minutes with the help of the eye rinse facility present. In case animal gets into the mouth , Rinse the mouth straight away with water or physiological salt. For more information you can contact the BSO Natasja Kisters, tel 81137 e-mail: n.kisters@maastrichtuniversity.nl Marjanne Markerink, tel. 82986 e-mail: m.markerink@maastrichtuniversity.nl Remaining relevant forms: see www.crispmaastricht.nl Accompanying Commercial Document Importexemption VWA Instruction on disinfection of laboratories Instruction on transportation of Biological samples Version 02, jan. 2012