2011 SOP - University of Florida
advertisement

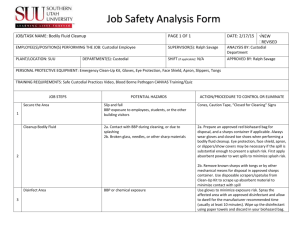
University Of Florida Bloodborne Pathogen Program Standard Operating Procedures Revised February 9, 2011 Updated (annually) ________ ________ ________ ________ ________ BBP Standard Operating Procedures Page 1 of 13 keep in lab University Of Florida Bloodborne Pathogen Program Standard Operating Procedures Please use the enclosed worksheets to create standard operating procedures (SOPs) for individual laboratories or work areas. These pages are intended to be photocopied and used as a means of compliance with the bloodborne pathogen standard. These worksheets will assist you in tailoring the Exposure Control Plan to your individual needs. Please note that they are to be used in conjunction with the UF Exposure Control Plan. Keep the completed worksheets with the Exposure Control Plan in a location that is accessible to your employees. EH&S will monitor individual laboratories for the presence of SOPs. There is no need to send the completed sheets to EH&S. Table of Contents Facility Identification Sheet ............................................................................................ 3 JOBS: Exposure Determination List .............................................................................. 4 TASKS: Exposure Determination List ........................................................................... 5 HBV Vaccine ..................................................................................................................... 6 Exposure Management ................................................................................................... 7 Protective Equipment Used ............................................................................................ 8 Decontamination Schedule ............................................................................................. 9 Engineering Controls Check ........................................................................................ 10 Biomedical Waste Plan .................................................................................................. 11 BBP Standard Operating Procedures Page 2 of 13 keep in lab University of Florida Exposure Control Plan Standard Operating Procedures Facility Identification Facility Name____Neurochemistry Core _________________________ Facility Room & Building #______ P2-32 & P2-41__Pharmacy Building 445_____ Mailing Address___JHMC Box 10487______________________________________ Phone Number______273-6311 or 846-1354_________________________________ Plan Prepared By________Dr. William J. Millard__________ Date____ February 18, 2009______________________________ Date of Review/Update_________ February 9, 2011___________ ____________________________________________________________ Signature of supervisor __William J. Millard_________________________________________________ Name of supervisor (PLEASE PRINT OR TYPE) _Pharmacodynamics_______________________________ Department (PLEASE PRINT OR TYPE) BBP Standard Operating Procedures Page 3 of 13 keep in lab Exposure Determination List Jobs The following persons and job classifications in this facility may have contact with blood or other potentially infectious materials. These people are in the UF BBP program. (Please use official UF job classification titles). NAME JOB CLASSIFICATION William J. Millard__________________ ___Professor_____________________ Yun-Ju He_________________________ ___Senior Biological Scientist_______ __________________________________ _________________________________ __________________________________ _________________________________ __________________________________ __________________________________ __________________________________ __________________________________ __________________________________ __________________________________ __________________________________ __________________________________ _________________________________ _________________________________ _________________________________ _________________________________ _________________________________ _________________________________ _________________________________ _________________________________ __________________________________ __________________________________ __________________________________ __________________________________ __________________________________ __________________________________ __________________________________ __________________________________ __________________________________ _________________________________ _________________________________ _________________________________ _________________________________ _________________________________ _________________________________ _________________________________ _________________________________ _________________________________ __________________________________ __________________________________ __________________________________ _________________________________ _________________________________ _________________________________ BBP Standard Operating Procedures Page 4 of 13 keep in lab Exposure Determination List Tasks The following procedures performed in this facility may result in occupational exposures to blood or other potentially infectious materials. (Please list all tasks in which exposure may occur). Radio-immunoassay Procedures______ _________________________________ Sample Pipetting and aliquotting______ _________________________________ Enzyme linked Immunosorbant Assay_ ___________________________________ ___________________________________ ___________________________________ ___________________________________ ___________________________________ ___________________________________ ___________________________________ ___________________________________ _________________________________ _________________________________ _________________________________ _________________________________ _________________________________ _________________________________ _________________________________ _________________________________ _________________________________ ___________________________________ ___________________________________ ___________________________________ ___________________________________ ___________________________________ ___________________________________ ___________________________________ ___________________________________ ___________________________________ ___________________________________ _________________________________ _________________________________ _________________________________ _________________________________ _________________________________ _________________________________ _________________________________ _________________________________ _________________________________ _________________________________ ___________________________________ ___________________________________ _________________________________ _________________________________ BBP Standard Operating Procedures Page 5 of 13 keep in lab Hepatitis B Vaccine Write in the names of your employees, with the date they were offered the hepatitis B vaccine. The following at-risk employees have been offered the hepatitis B vaccine free of charge: Name Date provided Date declined William J. Millard_______________________ 1/94, 2/94, 7/94 ___________ Yun-Ju He______________________________ 6/93, 10/94, 8/96 ___________ _______________________________________ ______________ ___________ _______________________________________ ______________ ___________ _______________________________________ ______________ ___________ _______________________________________ ______________ ___________ _______________________________________ ______________ ___________ _______________________________________ ______________ ___________ _______________________________________ ______________ ___________ _______________________________________ ______________ ___________ _______________________________________ ______________ ___________ _______________________________________ ______________ ___________ _______________________________________ ______________ ___________ _______________________________________ ______________ ___________ _______________________________________ ______________ ___________ _______________________________________ ______________ ___________ _______________________________________ ______________ ___________ _______________________________________ ______________________________ BBP Standard Operating Procedures Page 6 of 13 keep in lab Exposure Management In the event of an exposure to human blood or other potentially infectious materials, employees of this facility shall do the following (please fill in specifics for this work area): 1. Wash skin and wound exposures with soap and water: Wash for at least 5 minutes with alternate soap and water rinse. 2. Wash eye and mucous membrane exposures in running water for 15 minutes: Eye wash station in the lab 3. Report exposures to the supervisor: Dr. William J. Millard, Room 4-334, HPNP , Phone # 273-6311 or 8718385 4. Individuals exposed to human blood or other potentially infectious materials shall go immediately (within one hour) to the following location for treatment: (866) 477-6824 in the Gainesville area Only. This Needle Stick hotline is manned 24 hours a day. You will be directed from there were to go for further treatment 5. In All other Locations: Individuals exposed to human blood or other potentially infectious materials shall go immediately (within one hour) to the following location for treatment. BBP Standard Operating Procedures Page 7 of 13 keep in lab Protective Equipment (by task) Task Personal Protective Equipment Used Aliquotting and pipetting samples Gloves goggles, gowns Vortexing Samples Gloves goggles, gowns Centrifugation of samples Gloves goggles, gowns Aspiration of Samples Gloves goggles, gowns BBP Standard Operating Procedures Page 8 of 13 keep in lab Equipment/Worksite Decontamination Schedule for Neurochemistry Core RIA Facility Facility Pharmacy Building #455 – Room P2-41 Location Equipment Schedule Pipettors End of experimental day Gamma Counter End of experimental day Assay Racks End of experiment Pipette tips End of experiment Centrifuges End of experiment RIA disposables End of experiment Lab Benches End of experiment Aspirator End of experiment BBP Standard Operating Procedures Procedure Wipe outside and clean inside with 1:100 bleach solution. Wipe outside with detergent solution Soak overnight in 1:10 bleach solution, wash with count-off detergent Discard in red biohazard bag Wipe outside and clean inside with 1:100 bleach solution. Soak overnight in 1:10 bleach solution, discard in radioactive waste. Discard bench paper in red biohazard bag. Soak overnight in 1:10 bleach solution, discard liquid in radioactive waste. Page 9 of 13 keep in lab Engineering Controls Check for Neurochemistry Core RIA Facility Facility Pharmacy Building #455 – Room P2-41 Location Equipment Inspection Dates Action Handwashing facilities Monthly Refill supplies Sharps containers Monthly Empty if necessary Specimen containers Quarterly Check for leaks Regulated waste Monthly Check for leaks, empty if necessary Waste containers Monthly Check for leaks, empty if necessary Mechanical pipettes Monthly Check for contamination, clean if necessary BBP Standard Operating Procedures Page 10 of 13 keep in lab UF Biomedical Waste Plan For Neurochemistry Core RIA Facility Facility Pharmacy Building #455 – Room P2-41 Location This plan is based upon the UF Biological Waste Policy. It is designed to ensure compliance with all local, state, and federal regulations and guidelines concerning biological waste, including 29 CFR Part 1910.1030, the OSHA Bloodborne Pathogen rule, and Chapter 64E-16, Florida Administrative Code, Biomedical Waste rule. A. Biomedical Waste In this facility, the following items constitute biomedical waste: Gloves, disposable garments, test tubes, pipettes, needles and syringes used in preparation and analysis of blood or tissue samples from human or animal sources. B. Disposal Containers 1. Red plastic sharps containers shall be used for: needles, syringes, razor blades, scalpel blades 2. Red biohazard bags shall be used for: blood and tissue samples and any container or material in which is contaminated by the blood or tissue samples. 3. Cardboard biohazard boxes shall be used for: gloves, test tubes and pipette tips 4. Regular trash receptacle: paper waste, non-radioactive. BBP Standard Operating Procedures Page 11 of 13 keep in lab C. Personal Protective Equipment The following personal protective equipment shall be worn when handling, packaging and disposing of biomedical waste: Gloves protective eyewear, lab coat (disposable and cloth), mask (as necessary) D. Labeling All packages containing biohazardous/biomedical waste shall be labeled with indelible ink marker (i.e. Sharpie) as follows: 1. Biohazard bags: Investigators Name Phone Number Room Location 2. Sharps containers: Investigators Name Phone Number Room Location 3. Corrugated boxes (biomedical waste boxes): Investigators Name Phone Number Room Location E. Transport Transport of biomedical waste shall take place as follows: EH&S Waste Management Picks Materials Up and Properly Discards Materials following OSHA Guidelines BBP Standard Operating Procedures Page 12 of 13 keep in lab F. Training regarding biological waste shall take place as follows: Each member of the Neurochemistry Laboratory will be required to undergo annual training by Dr. Millard. This includes an update on all new OSHA and EH&S mandates and viewing of any videotapes that are appropriate. BBP Standard Operating Procedures Page 13 of 13 keep in lab