Go for the Gusto -Finding Your Threshold of Taste
advertisement

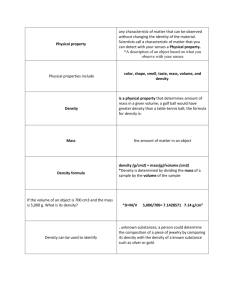
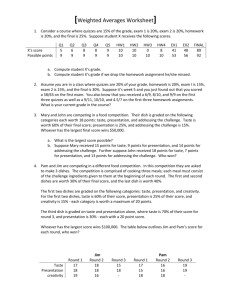
Name ______________________________ Chemistry Honors/Mrs. Lewton Go for the Gusto -Finding Your Threshold of Taste Reproduced and modified with permission from S. Stein (http://www.vivo.colostate.edu/hbooks/pathphys/digestion/pregastric/taste.html) The sense of taste is equivalent to excitation of taste receptors, and receptors for a large number of specific chemicals have been identified that contribute to the reception of taste. These include receptors for such chemicals as sodium, potassium, chloride, glutamate and adenosine. Despite this complexity, five types of tastes are commonly recognized: * Salty * Sour * Sweet * Bitter * Umami Perception of taste also appears to be influenced by thermal stimulation of the tongue. In some people, warming the front of the tongue produces a clear sweet sensation, while cooling leads to a salty or sour sensation. It should be noted that these tastes are based on human sensations and some comparative physiologists caution that each animal probably lives in its own "taste world". For animals, it may be more appropriate to discuss tastes as being pleasant, unpleasant or indifferent. A single chemical elicits none of these tastes. Also, there are thresholds for detection of taste that differ among chemicals that taste the same. For example, sucrose, 1-propyl-2 amino-4-nitrobenzene and lactose all taste sweet to humans, but the sweet taste is elicited by these chemicals at concentrations of roughly 10 mM, 2 uM and 30 mM respectively - a range of potency of roughly 15,000-fold. Substances sensed as bitter typically have very low thresholds. Examples of some human thresholds: Taste Substance Threshold for tasting Salty (NaCl ) 0.01 M Sour (HCl) 0.0009 M Sweet (Sucrose) 0.01 M Bitter (Quinine) 0.000008 M Umami (Glutamate) 0.0007 M http://www.edinformatics.com/math_science/science_of_cooking Each student is expected to: read through the background of this lab answer the pre-lab questions on separate paper write a flowchart in his/her lab notebook and construct data tables in your lab notebook Pre-Lab Questions: 1. Looking at the five recognized taste types, which is your favorite taste type? 2. Calculate the molar mass of the following compounds: a. Potassium dichromate b. Sodium hydroxide c. Calcium phosphate 3. Calculate the molarity of a solution that has 12.5 g of sodium chloride dissolved in distilled water to make a 100. mL solution. Purpose: The purpose of this experiment is to determine the lowest concentration of a substance dissolved in water, which can still be tasted – the taste threshold. We will specifically be working with sweetness. In addition, you will practice calculating molar mass, learn to make a solution of a desired concentration, learn to do a serial dilution and practice using Capstone to display your results as well as the results of the class. Materials: Table sugar (Sucrose) Water (preferably distilled) paper dixie cups (10) paper towels balance 1 ml dropper (2) PROCEDURE (working in pairs): 1. Calibrate a clean Dixie cup by adding 10 ml of water to it using your sterile 1 ml dropper. Use a pen to carefully mark the level of the water on the outside of the cup. 2. You will need to make a 1 M solution of sucrose, a disaccharide. http://wiki.answers.com/Q/How_would_you_prepare_a_1_mole_solution_of_sucrose_C12H22O11 What is the molecular formula for sucrose? http://www.edinformatics.com/math_science/science_of_cooking/taste_molecules.htm If the molecular formula is C12H22O11, then what is the mass of 1 mole of sucrose? _____ atoms C X _____ g per C atom = _____ g _____ atoms H X _____ g per H atom = _____g _____ atoms O X _____ g per O atom = _____ g total mass 1 mole sucrose = _____ g To make a 1 M solution, you need to add the molar mass of sucrose to enough water to make 1 L of solution. Since you don't need 1 L of solution, you should make just what you need. If you want to make just 10 ml of 1M solution, how would you do that? Add _____g sucrose (molar mass/100) to enough water to make 10 ml. *Measure out that mass of sucrose in a clean Dixie cup that you have calibrated to 10 ml. Carefully use the dropper to add 8 ml of water to the cup. Stir with the dropper until the sugar dissolves. Add enough extra water to make 10 ml of your solution. You have just made a 1M sucrose solution! 3. You will now need to make a series of dilutions until you reach a dilution just below the typical threshold for sucrose. A series of dilutions is typically called a serial dilution. Label your cups so that you have one for each of the solutions that you will make. With a clean, sterile 1 mL graduated dropper, measure out 2 mL of water into a clean cup. Add 2 ml of your 1 M solution. This will give you a .5 M sucrose solution. Add 1 ml of your 1 M solution to 9 ml of water. You have just made a .1 M sucrose solution. 4. You now need to repeat the above steps using the correct stock concentration in order to make all of the dilutions listed in your data table. Use the chart below to help you make these solutions. _____ ml .1 M _____ ml water .05 M solution _____ ml .1 M _____ ml water .01 M solution _____ ml .01 M _____ ml water .005 M solution _____ ml .01 M _____ ml water ,001 M solution 5. Label another complete set of Dixie cups with the concentrations that you have just made. Pour approximately half of the solution into each of the new cups. You should each have a complete set of solutions with varying concentrations. 6. Rinse your mouth with plain water. Decide with your partner in which order you will test the solutions and for how long will the solution remain in your mouth. For each solution, swirl small volumes of each of the desired concentrations in your mouth for your pre-determined set time. 7. Rinse your mouth and repeat with the other solutions until you can no longer taste "sweet". The lowest concentration at which you can still taste the sweetness is your approximate taste threshold. Results: CONCENTRATION SWEET 1.0 M TASTE / Yes TASTE / No .50 M .10 M .05 M .01 M .005 M .001 M What is your taste threshold for sweet (sucrose)? ____________ M sucrose 8. Using the following diagram, determine the molecular formula for sucralose (Splenda): 9. Repeat the lab procedure for sucralose and determine your individual taste thresholds. Class Data: 1. Design a data table to collect class data. Calculate the average and range. You might also want to consider: Males vs. Females? Current Allergies or Cold? 2. Construct a bar graph showing the number of people at each threshold. Explain your graph in a caption directly under the graph. Discussion Questions: 1. What is your taste threshold for sweet? How does this compare to known values? How does this compare to your classmates? (Use your graph to help you answer this) 2. Are your thresholds for all tastes the same? Why or why not? 3. What factors might account for differences in taste thresholds between people? 4. Will your taste thresholds be more likely to be higher or lower when you are fifty years old? Why? see also: http://www.cf.ac.uk/biosi/staffinfo/jacob/ Teaching Notes: 1. You can extend this lab by next having student make appropriate solutions for NaCl, vinegar (already diluted acetic acid - 4-8% http://en.wikipedia.org/wiki/Acetic_acid), and quinine (tonic water concentration = 83 mg/L (ppm) - http://en.wikipedia.org/wiki/Quinine ; http://en.wikipedia.org/wiki/Tonic_water). 2. Try thresholds with different temperature solutions 3. connection to taste bud lab 4. MW sucrose = 342.30 Some other cool taste facts: Thresholds: are influenced by temperature, and viscosity. Also they vary across the tongue. We have a lot of variability to SOME tastes (vanilla, to which some are almost "taste blind," genetically) and very little variability to others (salt). Caffeine involves a LOT of variability. Taste adaptation: occurs in seconds, e.g. at low concentrations by 3 seconds, at higher concentrations by 10 seconds. .Recovery: virtually complete by 10 seconds for threshold. FAST EATING DIMINISHES OVERALL TASTINESS Cross-adaptation: Adaptation to one taste (e.g. a salty food) incurring adaptation to others too (other salty foods). Potentiation: Adaptation to one taste (e.g. sour) making us MORE sensitive to another (e.g sweet). ex. sodium laurel sulphate in TOOTHPASTE makes the citric acid in ORANGE JUICE taste very bitter (as well as slightly sour) rather than just slightly sour. Cross adaptation and potentiation can affect tastes of ordinary tap water e.g. it tastes sweet after eating artichokes. Resources: http://www.cf.ac.uk/biosi/staffinfo/jacob/