Physical Science Final Review
advertisement

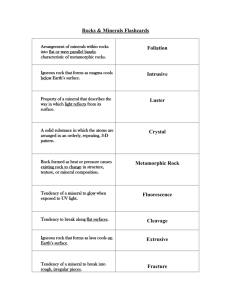
Physical Science Final Review Earth Science Chapter 28: Earthquakes Fault: break in the earth’s crust along a transform boundary Plate Boundaries: boundaries along which plates collide Transform: plates boundaries slide past Convergent: plate boundaries collide Divergent boundaries: plate boundaries separate Relative dating: a way to put events in order of which they happened: crosscutting, inclusions, original horizontality, lateral continuity, fossils(faunal succession), superposition Fossil: remains of prehistoric life Tsunami: underwater earthquakes, volcanic eruptions or landslides cause violent displacement of the sea floor Kinetic energy: energy of motion (movement) Potential energy: stored energy Epicenter: the point on the surface directly above the focus Focus: exact point below the surface where the earthquake started Richter Scale: measures the amount of energy released using a scale of 1-10 P-waves: are the first waves to arrive at a seismograph station. Are the fastest form of wave, sometimes called compression waves S-waves: arrive after the primary waves at the seismograph station. Can travel through solids but not liquids Plate Tectonics: theory of plate movement that explains the formations of mountains, ridges, earthquakes, volcanoes, trenches, etc Trench: feature where oceanic and continental crust converge Mid-ocean Ridge: largest ridge in our lab created by divergent plate boundaries Geological time scale: Earth’s history divided into eras What is Pangaea & evidence for Pangaea? Pangaea is the theory that all continents were once part of a single land mass o Ocean ridge rocks are younger than the surrounding ocean rock o The continents appear to fit together o Rock & fossil correlation is found where the continents appear to fit together What feature is found where oceanic and continental crust converge? • Subduction: oceanic plate dives below the continental crust • A deep oceanic trench marks the boundary between a subducting and an overriding plate at a convergent boundary. What feature is found where continental plates converge? Mountains or mountain chains How many seismograph stations are needed to locate an epicenter? 3 A 436g sample of a radioisotope has a half-life of 3.4 days. How much of the sample will remain after 17.0 days? 5 half lives 436g 218g 109 g 54.5 g 27.25 g 13.625g = 14g How much of a 352g sample of a radioisotope will remain after 4 half-lives? 352g 176g 88 g 44 g 22g = 22.0g After 18 days the radioactivity of a sample decreases from 2500 counts to 625 counts. What is the half-life of this sample? 2500c 1250c 625c = 2 half lives 18days / 2 half lives = 9days / 1 half life The half-life of a radioisotope is 2.6 days. What time must elapse for the radiation to be reduced to 12.5% of its original level? 100% 50% 25% 12.5% = 3 half lives 3 half lives x 2.6 days _______ = 7.8 days 1 half life Chapter 29.3: Rocks & Minerals: Relative Dating Concepts: Understand how to identify a mineral, igneous rock, sedimentary rock, or metamorphic rock. Igneous rock – may be frothy, glassy, show conchoidal fracture, formed from lava or magma cooling Sedimentary – formed by cementation and compaction, may have fossils or clasts Metamorphic – formed by heat and pressure, may have foliations Mineral – “pure” inorganic substance, building blocks of rocks What is the difference between a mineral and a rock? Mineral is a solid, naturally-occurring object with a defined chemical composition, inorganic and has a crystalline structure. Rock is a naturally formed consolidated solid mixture containing minerals, rock fragments, or volcanic glass What do you use to identify a mineral? Physical Properties: Cleavage ◦ Minerals break along planes that cut across relatively weak chemical bonds, a smooth, flat surface is created. ◦ Most minerals (except metals) have one or more cleavage planes that also help in determining their identity. Fracture ◦ irregular break ◦ Some minerals do not split along well-defined flat surfaces. ◦ In such cases, a mineral will break unevenly. Hardness ◦ The physical property that measures resistance to scratching ◦ Mohs Hardness Scale ◦ developed in 1812 by Friedrick Mohs (an Austrian mineral expert) as a method to identify minerals. ◦ Identify a mineral’s place on the hardness scale by whether it can scratch another mineral Luster ◦ The way a mineral reflects light is the physical property known as luster. ◦ Metallic and nonmetallic. ◦ Metallic luster minerals reflect light in a way that a metal surface might. ◦ Nonmetallic luster, includes minerals that shine like glass or appear earthy or waxy. Streak ◦ The color of mineral in powdered form is called streak. Crystal Shape ◦ The orderly internal arrangement of atoms in a mineral often is indicated by its external crystal shape. What do you use to identify an igneous rock? Igneous ◦ - crystals intersecting at angles ◦ -size of the grain What do you use to identify a sedimentary rock? ◦ -layers of rock pieces What do you use to identify a metamorphic rock? ◦ -pressure created results in lines ◦ -pressure and heat create grains in foliation (wavy patterns) ◦ -hardest of the 3 rocks Know the difference between intrusive & extrusive. Include temperature, time, and grain size. ◦ Intrusive Igneous rocks Formed from magma which cools and solidifies below Earth’s surface Cooling and solidification take a long time resulting in large visible crystals Coarse-grained like granite Granite is mostly found in the continents ◦ Extrusive Igneous rocks Formed from lava on or above Earth’s surface Cooling and solidification takes place relatively quickly resulting in very small crystals Fine-grained like basalt Basalt is in the ocean floor Identify and define the different types of relative dating techniques. (Superposition, original horizontality, inclusions, faunal succession, intrusive relationships, lateral continuity.) Superposition – older layers on bottom, youngest layers on top Original Horizontality – sediment forms horizontal layers Inclusions – rocks (clasts) included in a rock are older than the rock around them Faunal Succession – Fossils included in rock layers are the same age as the rocks they are in. Intrusive relationships – veins of rock and faults are younger than the rock they cut through. Lateral continuity – rock layers extend in all directions until they are disturbed by another formation. Chapter 24 & 26: Atmosphere & Water Cycle Barometer: instrument to measure pressure Conduction: transfer of heat by direct contact Convection: transfer of heat through a medium or indirectly Transpiration: water released into the atmosphere from plants (evaporation of water from plants) Evaporation: water molecules speed up enough to break bonds and phase change into a gas. Run off: water that flows over land before reaching surface water Ground water: water that collects underground Clouds: place where condensation occurs Condensation: vapor molecules lose kinetic energy, slow down, reform bonds and phase change to a liquid Energy: comes from the sun in order to keep the water cycle going, light & heat in the form of radiation Know the diagrams of the water cycle. The diagram we took a quiz on & took notes on is on the final. Where does the majority of water come from? oceans Why is the water cycle necessary? There is very little water that is good for drinking What happens to pressure as you increase altitude? Pressure decreases What are the most prevalent gases in the atmosphere from greatest to least? Nitrogen, Oxygen, Hydrogen, water vapor What human activity has increased carbon dioxide most in the atmosphere? Fossil fuels (other contributors are pollution & refridgerants) List the layers of the atmosphere from closest to the earth to outer space? Troposphere Tropopause- boundary Stratosphere Stratopause- boundary Mesosphere Mesopause- boundary Thermosphere Chapter 27: Weather Coriolis Effect: The bending of air currents due to the Earth’s rotation Relative Humidity: amount of water vapor in air compared to maximum amount air can hold Sling psychrometer: measures relative humidity - 2 thermometers - 1 wet-bulb (wet cloth) - 1 dry-bulb - wet bulb: water evaporates from cloth shows ↓ cloth - dry bulb: temperature does not change - air is dry water evaporates quickly, difference between wet and dry bulb is large - air has a large water amount – little water evaporates from wet bulb; difference is small - no evaporation- same temperature *difference indicates the amount of water vapor in air. Absolute Humidity: - measure of amount of water vapor in air & measured in grams per cubic meters (g/m3) - airs ability to hold water vapor depends on temperature of air - temperature ↑ capacity for water vapor ↑ Why does Earth have seasons? Earth is tilted on its axis which allows one hemisphere to get more radiation from the sun than the other hemisphere- thus changing the weather & giving us seasons. What happens to the sun’s radiation in our atmosphere? Some of the radiation is absorbed in the form of light and heat (which helps us to thrive) Some is reflected (emitted) out back into space as infrared radiation or heat. Read a relative humidity chart. Chemistry Atom: smallest unit of matter that cannot be easily broken down Electron: Tiny negatively charged particle attracted to the positive charges in the nucleus.- a lot of volume Proton: protons are positively charged and very close together in the nucleus- a lot of mass Neutron: Neutrons make the nucleus more stable by adding to the strong nuclear force, without adding positive, repulsive charges - a lot of mass Compound: two different atoms that are held together by a strong or weak chemical bond Chemical Formula: Element: one type of atom (matter) Molecule: two or more atoms that are held together by a strong chemical bond Ion: charged particle or atom (b/c lost or gained electrons) Reactants:chemical compounds that are combined (left side of the arrow) Products: the resultant chemical compounds (right side of the arrow) Isotope: atoms of the same element that have different numbers of neutrons. Anion: gains electrons & negatively charged ion Cation: loses electrons & positively charged ion Ionic: metal bonds nonmetal Covalent: nonmetal bonds nonmetal Acid: starts with hydrogen H1+ What properties do solids, liquids, and gases have? Solids: o A solid retains its volume and shape. o Vibrate o Least Kinetic Energy Liquids: Binary Acid: hydrogen & one other element (no oxygen) Oxyacid: hydrogen & polyatomic Coefficients: make the total number of atoms of each element the same for the reactants as for the products Physical Change: any change that does not affect the identity of the atoms or molecules in the material Chemical Change: results in the formation of new molecules as atoms are rearranged Mixture: contain more than one kind of matter and can be separated by physical means Substance: material composed of only one type of molecule (pure) &cannot be separated into different kinds of matter by physical means Homogenous: mixture is the same throughout Heterogenous: mixture has visibly different substances throughout. Valence electron: outer shell electrons in “s” & “p” columns Octet Rule: all elements want 8 valence shell electrons (Noble Gas Wannabe’s) Strong Nuclear Force: Attractive force between any two nuclear particles (protons, neutrons), Only acts over a very short distance & Overcomes the repulsive force between the positive protons Mass Number: The total number of protons and neutrons in the nucleus Atomic mass: Average mass of protons and neutrons combined. atomic mass units (amu) o o o Gases: o o o o A liquid has a definite volume, but no definite shape. Liquids flow to take the shape of the container. vibrate, rotate, translate A gas has no definite volume or shape. Gases expand to fill their containers. vibrate, rotate, translate Most Kinetic Energy List the states of matter in order of their kinetic energy from least to greatest. Solids liquids gases plasma Know the heating and cooling curve & Phase Diagram. -Know how to write chemical compound names and chemical formulas. CO carbon monoxide calcium iodide CaI2 CO2 carbon dioxide hydrogen chloride HCl nitrogen dioxide NO2 HBr(aq) hydrobromic acid N2O4 dinitrogen tetraoxide hydrogen sulfide H2S dinitrogen pentaoxide N2O5 Na2O sodium oxide PbO lead (II) oxide magnesium nitride Mg3N2 lead (IV) oxide PbO2 calcium phosphide Ca3P2 sulfur dioxide SO2 aluminum carbide Al4C3 SO3 sulfur trioxide LiH Lithium Hydride BCl3 boron trichloride magnesium sulfide MgS iron (II) chloride FeCl2 copper (I) oxide HI(aq) hydroiodic acid Hydronitric acid H3PO3 phosphorous acid Chlorous acid H3PO4 phosphoric acid Sulfurous acid Cu2O H3N(aq) HCLO2 H2SO3 HCl(aq) hydrochloric acid Hydrofluoric acid HF(aq) FeCl3 iron (III) chloride copper (II) oxide CuO KBr potassium bromide MnF7 manganese (VII) fluoride -Know how to balance equations and how to identify their “type”. _2_ZnS + _1_O2 _1_Li2O + _1_C3H8 + _5_O2 → _2_ZnO + _2_S _1_H2O → → _2_H2O + _1_ CH4 _3_CaO + _2_Al _2_LiOH _4_H2O Synthesis + _3_CO2 → _4_H2 + _1_CO2 → Single Replacement _1_Al2O3 + _3_Ca _______________ Combustion (not one we looked at) Single Replacement Name indicators of a chemical change. Formation of a Precipitate – insoluble solid Exothermic Reaction - Increase in temperature Formation of a gas without heating Endothermic Reaction - Decrease in temperature Change in color Evolution of Light without heating Change in smell Change in taste What is a physical change and give an example of one? Any change that does not affect the identity of the atoms or molecules in the material o Bending, Phase changes, breaking, heating, etc Identify the following as physical or chemical changes: Paper tearing Wood burning Ice melting Water boiling Reheating leftover food Digesting food physical chemical physical physical physical chemical Study hard and then have a great summer!!!!!!!!!