nuclear chem notes
advertisement

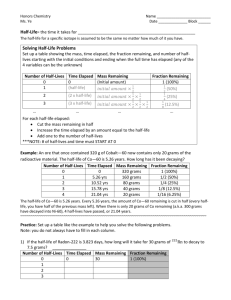
Nuclear Reactions There are __________ ways to identify elements by isotope. 1 – ____________ The element’s _______ is followed by its _____________. Ex: lead – 207, carbon-13, lithium-7… Give the name of the following isotopes: a. 10 p+, 10e-, 11 no c. 1 p+, 1e-, 0 no b. 20 p+, 18e-, 22 no d. 32p+ , 36 e- , 38no 2 – ________ ____________ The chemical symbol is “surrounded” by data. Mass Number Atomic Number Ex: 27 13 Charge Symbol Al This symbol shows the aluminum-27 isotope. There is no charge on this atom 13 p+ (atomic # is 13) 13 e- (no charge) Ex: 31 15 P + -3 15 p (atomic # is 15) 14 no (atomic mass = 27) This symbol shows the phosphorous-31 isotope. The charge on this anion is -3. 18 e- (-3 charge) 16 no (atomic mass = 31) Identify the name the #p+, #no, and # e- for the following: 40 20 +2 Ca Si 207 82 Pb 35 17 -1 Cl 4 2 He PA Nuclear Reactions Important Facts to KNOW; 1- Nuclei are unstable because there are ___ _______ _________________ . 2- There are four types of nuclear ______________ that are common in nuclear reactions. a. They are NOT elements or atoms, just forms of radiation. 3- ______________ is when one unstable nucleus splits into two or more pieces 4- ___________ is when two nuclei combine into one nuclei 5- Nuclear reactions also occur when two nuclei ________ , but then ___________ into two other nuclei. Type Symbol Mass Charge (amu) notes Neutron Alpha Beta Gamma To balance a nuclear reaction… Keep in mind the _____ __ ________________ __ _______ must still be observed. The ______ ___________ must sum to the same value on each side of the equation. The __________ __________ must sum to the same value on each side of the equation. Examples of different types of decay: Neutron Decay: 210 84 1 92U 238 19 9 209 Po 0 n + 84 Po Alpha decay: Beta Decay: Explain 90 Th + 2 234 4 +2 F +-1+ 10Ne 0 19 Complete following nuclear reactions. Are alpha, beta or neutron decays? a. 210 85 c. At ____ + -1 0 208 83 207 Bi ___ + 83 Bi b. 222 Rn 86 ___ + 2 4 +2 Nuclear Reactions Half-Life The length of a half-life describes ___________________________________________ The shorter the half-life, the ___________________________________________ The longer the half-life, the___________________________________________ amount Time (in half lives passed 400 grams to - initial observation starting time t1 - the first half life t2 - the same amount of time between to and t1 t3 - again, the same amount of time between to and t1... or between t1 and t2... t4 - again, the same amount of time between to and t1... or between t1 and t2... or t2 and t3 t5 - again, the same amount of time between to and t1... or between t1 and t2... or t2 and t3 ... or t3 and t4 t6 - again, the same amount of time between to and t1... or between t1 and t2... you get it... Graph the relationship between amount (in grams) and number of half lives passed 400 300 Amount (grams) 200 100 0 0 1 2 What type of relationship is this? 3 4 5 Number of half-lives 6 7 8 1) If we start with 48 atoms of a radioactive substance, how many would remain … a. After one half-life? c. after three half-lives? b. after two half-lives? d. after four half-lives? Use the following chart to answer questions 2-5 Radioactive Isotope Radon-222 Iodine-131 Radium-226 Carbon-14 Plutonium-239 Uranium-238 Half Life 4 days 8 days 1600 years 5730 years 24,120 years 4,470,000,000 years 2) If a sample starts with 8000 atoms of radium-226, how much would remain after 3,200 years? 3) If a sample starts with 20 grams of plutonium-239, how many grams would remain after 48,240 years? 4) If a sample was found with 60 atoms of radon-222, how many were there 12 days ago? 5) If a sample was found with 3.25 grams of iodine-131, what mass was there 32 days ago?