cape chemistry - WordPress.com
advertisement

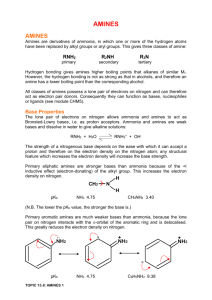
CAPE CHEMISTRY UNIT 2 AMINES MODULE 1 INTRODUCING AMINES This page explains what amines are, and what the difference is between primary, secondary and tertiary amines. It looks in some detail at their simple physical properties such as solubility and boiling points. Details of the chemical reactions of amines are described on separate pages. Note: This page only deals with amines where the functional group is not attached directly to a benzene ring. Aromatic amines such as phenylamine (aniline) are sufficiently different that they are covered in a separate section. Follow this link if you are mainly interested in phenylamine. What are amines? The easiest way to think of amines is as near relatives of ammonia, NH3. In amines, the hydrogen atoms in the ammonia have been replaced one at a time by hydrocarbon groups. On this page, we are only looking at cases where the hydrocarbon groups are simple alkyl groups. The different kinds of amines Amines fall into different classes depending on how many of the hydrogen atoms are replaced. Primary amines In primary amines, only one of the hydrogen atoms in the ammonia molecule has been replaced. That means that the formula of the primary amine will be RNH2 where "R" is an alkyl group. Examples include: Naming amines can be quite confusing because there are so many variations on the names. For example, the simplest amine, CH3NH2, can be called methylamine, methanamine or aminomethane. The commonest name at this level is methylamine and, similarly, the second compound drawn above is usually called ethylamine. Where there might be confusion about where the -NH2 group is attached to a chain, the simplest way of naming the compound is to use the "amino" form. For example: Secondary amines In a secondary amine, two of the hydrogens in an ammonia molecule have been replaced by hydrocarbon groups. At this level, you are only likely to come across simple ones where both of the hydrocarbon groups are alkyl groups and both are the same. For example: There are other variants on the names, but this is the commonest and simplest way of naming these small secondary amines. Tertiary amines In a tertiary amine, all of the hydrogens in an ammonia molecule have been replaced by hydrocarbon groups. Again, you are only likely to come across simple ones where all three of the hydrocarbon groups are alkyl groups and all three are the same. The naming is similar to secondary amines. For example: Physical properties of amines Boiling points The table shows the boiling points of some simple amines. type formula boiling point (°C) primary CH3NH2 -6.3 primary CH3CH2NH2 16.6 primary CH3CH2CH2NH2 48.6 secondary (CH3)2NH 7.4 tertiary (CH3)3N 3.5 We will need to look at this with some care to sort out the patterns and reasons. Concentrate first on the primary amines. Primary amines It is useful to compare the boiling point of methylamine, CH3NH2, with that of ethane, CH3CH3. Both molecules contain the same number of electrons and have, as near as makes no difference, the same shape. However, the boiling point of methylamine is -6.3°C, whereas ethane's boiling point is much lower at -88.6°C. The reason for the higher boiling points of the primary amines is that they can form hydrogen bonds with each other as well as van der Waals dispersion forces and dipole-dipole interactions. Note: If you aren't happy about intermolecular forces (including van der Waals dispersion forces and hydrogen bonds) then you really ought to follow this link before you go on. The next bit won't make much sense to you if you aren't familiar with the various sorts of intermolecular forces. Use the BACK button on your browser to return to this page. Hydrogen bonds can form between the lone pair on the very electronegative nitrogen atom and the slightly positive hydrogen atom in another molecule. The hydrogen bonding isn't as efficient as it is in, say, water, because there is a shortage of lone pairs. Some slightly positive hydrogen atoms won't be able to find a lone pair to hydrogen bond with. There are twice as many suitable hydrogens are there are lone pairs. The boiling points of the primary amines increase as you increase chain length because of the greater amount of van der Waals dispersion forces between the bigger molecules. Secondary amines For a fair comparison you would have to compare the boiling point of dimethylamine with that of ethylamine. They are isomers of each other - each contains exactly the same number of the same atoms. The boiling point of the secondary amine is a little lower than the corresponding primary amine with the same number of carbon atoms. Secondary amines still form hydrogen bonds, but having the nitrogen atom in the middle of the chain rather than at the end makes the permanent dipole on the molecule slightly less. The lower boiling point is due to the lower dipole-dipole attractions in the dimethylamine compared with ethylamine. Tertiary amines This time to make a fair comparison you would have to compare trimethylamine with its isomer 1-aminopropane. If you look back at the table further up the page, you will see that the trimethylamine has a much lower boiling point (3.5°C) than 1-aminopropane (48.6°C). In a tertiary amine there aren't any hydrogen atoms attached directly to the nitrogen. That means that hydrogen bonding between tertiary amine molecules is impossible. That's why the boiling point is much lower. Solubility in water The small amines of all types are very soluble in water. In fact, the ones that would normally be found as gases at room temperature are normally sold as solutions in water - in much the same way that ammonia is usually supplied as ammonia solution. All of the amines can form hydrogen bonds with water - even the tertiary ones. Although the tertiary amines don't have a hydrogen atom attached to the nitrogen and so can't form hydrogen bonds with themselves, they can form hydrogen bonds with water molecules just using the lone pair on the nitrogen. Solubility falls off as the hydrocarbon chains get longer noticeably so after about 6 carbons. The hydrocarbon chains have to force their way between water molecules, breaking hydrogen bonds between water molecules. However, they don't replace them by anything as strong, and so the process of forming a solution becomes less and less energetically feasible as chain length grows. Smell The very small amines like methylamine and ethylamine smell very similar to ammonia - although if you compared them side by side, the amine smells are slightly more complex. As the amines get bigger, they tend to smell more "fishy", or they smell of decay. If you are familiar with the smell of hawthorn blossom (and similarly smelling things like cotoneaster blossom), this is the smell of trimethylamine - a sweet and rather sickly smell like the early stages of decaying flesh. AMINES AS BASES This page looks at the reactions of amines as bases. Their basic properties include the reactions with dilute acids, water and copper(II) ions. It only deals with amines where the functional group is not attached directly to a benzene ring. Aromatic amines such as phenylamine (aniline) are much weaker bases than the amines discussed on this page and are dealt with separately on a page specifically about phenylamine. If you are interested in phenylamine, read this page first and then follow the link at the bottom. The basic properties of amines We are going to have to use two different definitions of the term "base" in this page. A base is a substance which combines with hydrogen ions. This is the Bronsted-Lowry theory. an electron pair donor. This is the Lewis theory. Note: If you aren't familiar with either of these terms, you should follow this link to a page about theories of acids and bases. Use the BACK button on your browser to return to this page when you are confident about these terms. The easiest way of looking at the basic properties of amines is to think of an amine as a modified ammonia molecule. In an amine, one or more of the hydrogen atoms in ammonia has been replaced by a hydrocarbon group. Replacing the hydrogens still leaves the lone pair on the nitrogen unchanged - and it is the lone pair on the nitrogen that gives ammonia its basic properties. Amines will therefore behave much the same as ammonia in all cases where the lone pair is involved. The reactions of amines with acids These are most easily considered using the Bronsted-Lowry theory of acids and bases - the base is a hydrogen ion acceptor. We'll do a straight comparison between amines and the familiar ammonia reactions. A reminder about the ammonia reactions Ammonia reacts with acids to produce ammonium ions. The ammonia molecule picks up a hydrogen ion from the acid and attaches it to the lone pair on the nitrogen. If the reaction is in solution in water (using a dilute acid), the ammonia takes a hydrogen ion (a proton) from a hydroxonium ion. (Remember that hydrogen ions present in solutions of acids in water are carried on water molecules as hydroxonium ions, H3O+.) If the acid was hydrochloric acid, for example, you would end up with a solution containing ammonium chloride - the chloride ions, of course, coming from the hydrochloric acid. You could also write this last equation as: . . . but if you do it this way, you must include the state symbols. If you write H+ on its own, it implies an unattached hydrogen ion - a proton. Such things don't exist on their own in solution in water. If the reaction is happening in the gas state, the ammonia accepts a proton directly from the hydrogen chloride: This time you produce clouds of white solid ammonium chloride. The corresponding reactions with amines The nitrogen lone pair behaves exactly the same. The fact that one (or more) of the hydrogens in the ammonia has been replaced by a hydrocarbon group makes no difference. For example, with ethylamine: If the reaction is done in solution, the amine takes a hydrogen ion from a hydroxonium ion and forms an ethylammonium ion. Or: The solution would contain ethylammonium chloride or sulphate or whatever. Alternatively, the amine will react with hydrogen chloride in the gas state to produce the same sort of white smoke as ammonia did - but this time of ethylammonium chloride. These examples have involved a primary amine. It would make no real difference if you used a secondary or tertiary one. The equations would just look more complicated. The product ions from diethylamine and triethylamine would be diethylammonium ions and triethylammonium ions respectively.