Net Ionic Equation Worksheet
advertisement

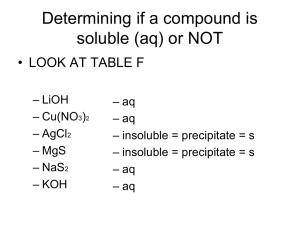
Name: _______ Per: _____ Date: ________________ Net Ionic Equations 11.3 – Reactions in Aqueous Solution (pages 342-344) 1. What does the symbol (aq) represent? (refer to your chemical reactions notes) 2. What is a complete ionic equation? Give an example 3. When ions do not change on either side of the equation what should you do to simplify? Why? 4. Define a spectator ion. 5. What is a net ionic equation? Give an example. 6. What is a precipitate? 7. Look at Table 11.3. Re-write these solubility rules into your own words. (ie. What are alkali metals? Look up and know any terms you are not sure about) Steps for Writing Net Ionic Equations 1. Determine if each compound is soluble (dissolved) or insoluble (solid) in water using the solubility rules. AgNO3 (aq) + NaCl (aq) AgCl (s) + NaNO3 (aq) (Rule number 4 says that chlorides are soluble except when paired with Silver, Lead, or Mercury) 2. Write a complete ionic equation. Write the soluble compounds as ions with their proper charges. Ag+ + NO3- + Na+ + Cl- AgCl(s) + Na+ + NO33. Eliminate the spectator ions. Ag+ + Cl- AgCl(s) (net ionic equation) Net Ionic Equation Practice Write balanced net ionic equations for each of the following reactions. Assume all reactions occur in aqueous solution. 1. NaCl(aq) + Pb(NO3)2(aq) PbCl2(s) + NaNO3(aq) 2. Na2CO3(aq) + FeCl2(aq) FeCO3(s) + NaCl(aq) 3. Mg(OH)2(aq) + HCl(aq) MgCl2(aq) + H2O(l) 4. K2(C2O4)(aq) + CaCl2(aq) KCl(aq) + Ca(C2O4)(s) 5. (NH4)3PO4(aq) + Zn(NO3)2(aq) NH4NO3( 6. LiOH(aq) + VCl3(aq) LiCl( 7. Na2CO3(aq) + HCl(aq) NaCl( 8. Mg(NO3)2(aq) + Na2CrO4(aq) NaNO3( 9. FeCl3(aq) + Mg(s) MgCl2(aq) + Fe(s) 10 Zr(OH)4(s) + HNO3(aq) Zr(NO3)4( 11. Na2SO3(s) + HCl(aq) NaCl( 12. BaBr2(aq) + Na2SO4(aq) 13. AgNO3(aq) + MgI2(aq) 14. (NH4)2C2O4(aq) + Al(ClO4)3(aq) 15. Ni(NO3)2(aq) + NaOH(aq) ) + V(OH)3( ) ) + CO2( )+ + H2O( ) + Zn3(PO4)2( ) )+ ) H2O( + MgCrO4( H2O( ) ) ) + SO2(g) ) )