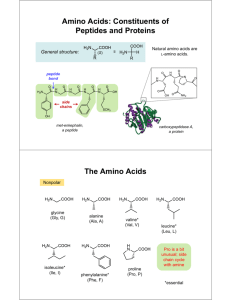
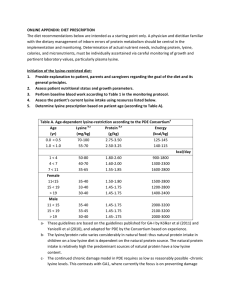
Using titration curves to determine pKa values
advertisement
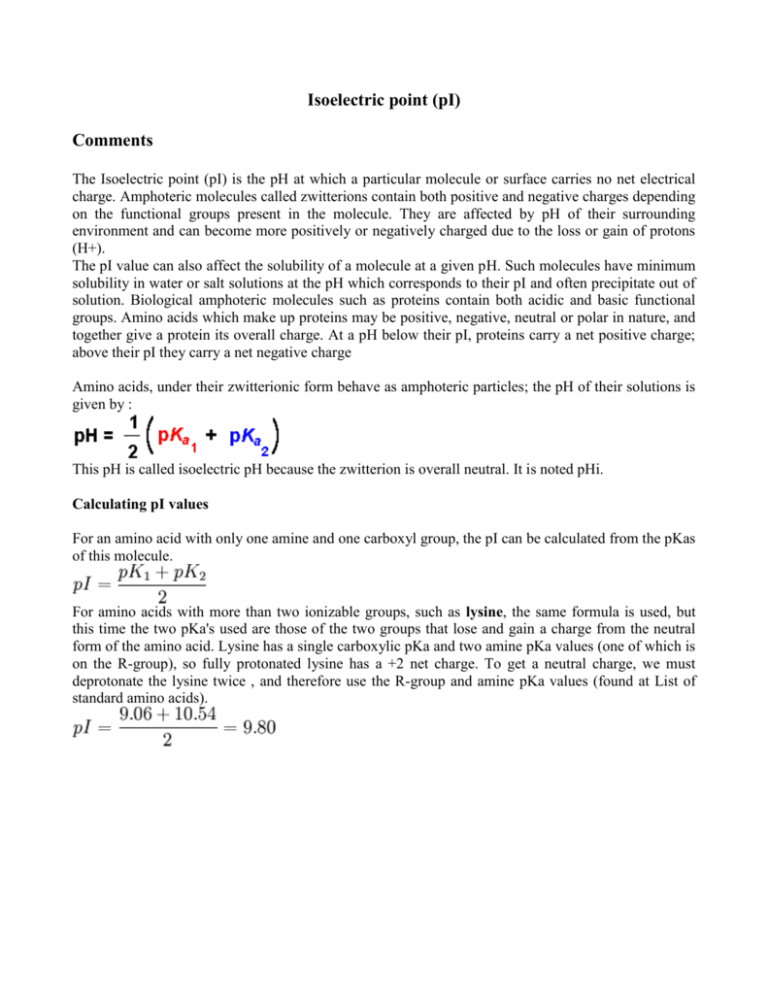
Isoelectric point (pI) Comments The Isoelectric point (pI) is the pH at which a particular molecule or surface carries no net electrical charge. Amphoteric molecules called zwitterions contain both positive and negative charges depending on the functional groups present in the molecule. They are affected by pH of their surrounding environment and can become more positively or negatively charged due to the loss or gain of protons (H+). The pI value can also affect the solubility of a molecule at a given pH. Such molecules have minimum solubility in water or salt solutions at the pH which corresponds to their pI and often precipitate out of solution. Biological amphoteric molecules such as proteins contain both acidic and basic functional groups. Amino acids which make up proteins may be positive, negative, neutral or polar in nature, and together give a protein its overall charge. At a pH below their pI, proteins carry a net positive charge; above their pI they carry a net negative charge Amino acids, under their zwitterionic form behave as amphoteric particles; the pH of their solutions is given by : This pH is called isoelectric pH because the zwitterion is overall neutral. It is noted pHi. Calculating pI values For an amino acid with only one amine and one carboxyl group, the pI can be calculated from the pKas of this molecule. For amino acids with more than two ionizable groups, such as lysine, the same formula is used, but this time the two pKa's used are those of the two groups that lose and gain a charge from the neutral form of the amino acid. Lysine has a single carboxylic pKa and two amine pKa values (one of which is on the R-group), so fully protonated lysine has a +2 net charge. To get a neutral charge, we must deprotonate the lysine twice , and therefore use the R-group and amine pKa values (found at List of standard amino acids).