The halogens
advertisement

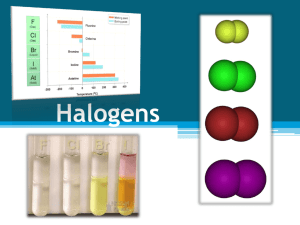
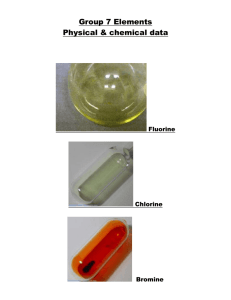
The halogens fluorine Atomic fluorine is univalent and is the most chemically reactive and electronegative of all the elements. In its elementally isolated (pure) form, fluorine is a poisonous, pale, yellowish brown gas, with chemical formula F2. Like other halogens, molecular fluorine is highly dangerous; it causes severe chemical burns on contact with skin. Pure fluorine (F2) is a corrosive pale yellow or brown gas that is a powerful oxidizing agent. It is the most reactive and most electronegative of all the elements (4.0), and readily forms compounds with most other elements. It has an oxidation number -1, except when bonded to another fluorine in F2 which gives it an oxidation number of 0. Fluorine even combines with argon, krypton, xenon, and radon. Even in dark, cool conditions, fluorine reacts explosively with hydrogen. It is so reactive that metals, and even water, as well as other substances, burn with a bright flame in a jet of fluorine gas. It is far too reactive to be found in elemental form. In moist air it reacts with water to form also-dangerous hydrofluoric acid. Chlorine In its common elemental form (Cl2 or "dichlorine") under standard conditions, it is a pale green gas about 2.5 times as dense as air. It has a disagreeable, suffocating odor that is detectable in concentrations as low as 3.5 ppm and is poisonous. Chlorine is a powerful oxidant and is used in bleaching and disinfectants. As a common disinfectant, chlorine compounds are used in swimming pools to keep them clean and sanitary. In the upper atmosphere, chlorine based molecules have been implicated in the destruction of the ozone layer. Chlorine gas, also known as bertholite, was first used as a weapon in World War I by Germany on April 22, 1915 in the Second Battle of Ypres. As described by the soldiers it had a distinctive smell of a mixture between pepper and pineapple. It also tasted metallic and stung the back of the throat and chest. Chlorine gas is diatomic, with the formula Cl2. It combines readily with all elements except O2 and N2 and the noble gases bromine Bromine is the only liquid nonmetallic element at room temperature and one of only six elements on the periodic table that are liquid at or close to room temperature. The pure chemical element has the physical form of a diatomic molecule, Br2. It is a dense, mobile, reddish-brown liquid, that evaporates easily at standard temperature and pressures to give a red vapor (its color resembles nitrogen dioxide) that has a strong disagreeable odor resembling that of chlorine. Bromine is a halogen, and is less reactive than chlorine and more reactive than iodine. Bromine is slightly soluble in water, and highly soluble in carbon disulfide, aliphatic alcohols (such as methanol), and acetic acid. It bonds easily with many elements and has a strong bleaching action. Elemental bromine is toxic and causes burns. As an oxidizing agent, it is incompatible with most organic and inorganic compounds. iodine Chemically, iodine is the least reactive of the halogens, and the most electropositive halogen after astatine. Iodine is primarily used in medicine, photography and dyes. It is required in trace amounts by most living organisms. As with all other halogens (members of Group VII in the Periodic Table), iodine forms diatomic molecules, and hence has the molecular formula of I2.







![[1] - Boswellsgmt](http://s3.studylib.net/store/data/006603407_1-fadfbce8d94050a9fb3c38a07d86e8ee-300x300.png)