FJCDLaryngectomyHopkins, Gross, Knutson, Candia 3-8
advertisement

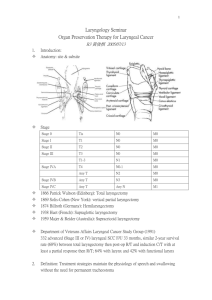
1 LARYNGECTOMY: INTRODUCTORY OVERVIEW Laryngectomy: An Introductory Overview Lynn E. Hopkins, B.S., Amy L. Gross, B.S., Nancy M. Knutson, B.S., and Christine L. Candia, M.S. Nova Southeastern University Incidence and Epidemiology Laryngeal cancer occurs when malignant cells emerge in the tissues of the larynx with unlimited and rapid growth, invading and destroying the normal tissue (National Cancer Institute, 2003; Stemple, Glaze, & Klaben, 2000, p. 441). Early detection, proper diagnosis and treatment may allow some cancers to be permanently resolved, while others may be recurrent (American Cancer Society, 2005). Pou (2004) stated that cancer of the larynx is one of the more curable cancers of the upper aerodigestive tract, with an estimated overall 5 year survival rate of 68%. Without proper treatment, cancers of the larynx have the ability to metastasize to nearby lymph nodes, the back of the tongue or other parts of the throat and neck and to the lungs and more distant anatomy (National Cancer Institute, 2003). Incidence figures report some of the following. It was estimated that in 2005 approximately 9,880 people in the United States would have laryngeal cancer (American Cancer Society, 2005). Lewin (2004a) stated that less than 1% of laryngeal cancers developed in individuals under the age of 30 years of age. She also noted that it was expected that approximately 0.7% of reported cancer cases in 2003 were laryngeal cancer, which translated to about 9,500 new cases diagnosed in that year. The American Cancer Society estimated that approximately 10,270 people in the United States were diagnosed with 2 laryngeal cancer in 2003. The male to female ratio in the United States was estimated to be approximately 3:1 (Lewin, 2004a) or between 4 and 5 times more common in men than women (American Cancer Society, 2005). However, the American Cancer Society noted that as the two main risk factors, smoking and alcohol abuse, became more common in women, risk levels among women have increased. The five-year survival rate was estimated to be from 68% to 75% with a lower rate (less than 40%) in advanced stages III or IV, which indicate progressively more metastasis to the surrounding neck tissue (Lewin, 2004a; Stemple et al., 2000, p. 444). Classification Variations in the types of laryngeal cancers may be identified by the type of cells from which malignant tumors originate. Approximately 95% of all laryngeal cancers are squamous cell carcinoma, while the relatively rare types include verrucous carcinoma, adenocarcinoma, fibrosarcoma, chondrosarcoma, and spindle cell carcinoma (Pou, 2004; San Diego Center for Voice and Swallowing Disorders, 2004). Laryngeal cancer occurs in the supraglottal, glottal, or subglottal areas, most often beginning in the flat, thin, squamous cells that line the vocal folds and surrounding larynx and hypolarynx (National Cancer Institute, 2003; Pou, 2004). Tumors may also be transglottic, with the affected region spreading across at least two of the three possible areas (Mendenhall, Million, Stringer, & Cassisi, 1999; San Diego Center for Voice and Swallowing Disorders, 2004). Cancer of the larynx is staged according to the tumor-node-metastasis (TNM) system, which was developed by the American Joint Committee on Cancer (AJCC) (Mendenhall et al., 1999). As illustrated in Mendenhall et al.’s chart below, the T system identifies the tumor size, location, and the extent that the tumor has spread to other tissue. Additionally, N describes the extent of the lymph node metastasis while M identifies the absence or presence of distant metastasis (Stemple et al., 2000. p. 444). As 3 emphasized by the above authors, the staging system is a crucial component of diagnosis and treatment as it allows communication between professionals and indicates prognosis. Table 1 T-Stages in AJCC Staging System of Laryngeal Cancer (Mendenhall, et al., 1999) Tis Carcinoma in situ T1 Tumor confined to vocal cord(s) with normal mobility T1a Normal cord mobility T2b Impaired mobility T2 Supraglottic and/or subglottic extension and/or impaired vocal cord mobility T3 Tumor continued to larynx, with vocal cord fixation T4 Tumor invading cartilage and/or extending beyond larynx to involve oropharynx or soft tissues of neck Thekdi and Ferris (2002) suggested that symptoms of laryngeal cancer are directly related to the location of the lesion. For example, hoarseness, as a symptom of cancer on the vocal folds, may be detected at an early stage, whereas cancer above or below the vocal folds may not be found until a later stage (American Cancer Society, 2005). In fact, it is possible for subglottal cancer to develop without presenting any perceptible symptoms (Thekdi & Ferris, 2002). Risk Factors The two most common risk factors associated with laryngeal cancer are smoking and alcohol consumption (Altieri, Garavello, Bosetti, Gallus, & La Vecchia, 2005; American Cancer Society, 2005; Lee et al., 2005; Lefebvre et al., 2004; Roychowdhury, Roychowdhury, & Sen, 2005). Leon, Renaldo, Saffiotti, and Ferlito (2004) report that greater than 95% of patients with laryngeal cancer have a history of tobacco and/or alcohol consumption, while other, less significant risk factors include exposure to 4 pollutants and irritants such as second-hand smoke, fossil fuel, asbestos, diesel engine emissions, metal working fluids, rubber industry products, and bitumen. Chronic gastroesophageal reflux may place an individual at significant risk for developing laryngeal cancer (Qadeer, Colabianchi, & Vaezi, 2005), as may consumption of red or processed meat, eggs and sugars (Leon et al., 2004). Leon et al. also listed viral infections that have been associated with laryngeal cancer including certain serotypes of human papillomavirus, herpes simplex virus, and HIV. Genetic predisposition has also been shown to affect an individual’s susceptibility to laryngeal cancer as noted in Leon et al. who cited inherited differences in DNA repair systems, carcinogen metabolizing systems and cell cycle control systems as factors affecting an individual’s genetic susceptibility. Treatments of radiation have been associated with thyroid cancer and head and neck sarcoma, although there is no direct link between radiation and squamous cell carcinoma in the head and neck region (Leon et al., 2004). Medical Intervention Medical intervention begins with an evaluation which may include the following: case history, blood tests (for general health information) indirect mirror laryngoscopy (a mirror is used to view the laryngeal structures, coloration, vocal fold mobility and gag reflex), indirect flexible nasoendoscopy (a flexible scope with fiberoptic lights attached to a video camera, is passed through the nose to assess vocal fold movement), and indirect rigid endoscopy with stroboscopy (magnifies the laryngeal structures and views vocal fold movement using a rigid scope with a strobe light source, through the mouth) (American Cancer Society, 2005; Stemple et al. 2000, p. 443). If indicated, direct fiberoptic endoscopy or panendoscopy are performed under general anesthesia where biopsies (tissue samples) may be taken (American Cancer Society, 2005; Stemple et al. 2000, p. 443). 5 Additionally, imaging techniques such as computed tomography (CT – a series of x-ray pictures from different angles, combined into one image by a computer), magnetic resonance imaging (MRI – radio waves and magnets are used instead of x-rays), barium swallow (x-rays taken after the individual swallows liquid with barium to coat the inside of the throat), chest x-ray (checks for lung cancer and emphysema), and positron emission tomography scan (PET – radioactive sugars are taken which are absorbed by cancer cells and detected by a camera) may also be performed (Agada, Nix, Salvage, & Stafford, 2004; American Cancer Society, 2005; Castelijns, Hermans, van den Brekel, & Mukherji, 1998). Castelijns et al. concluded that in general, examination utilizing MRI appeared to be the optimal method compared to CT and other diagnostic methods given the examiners experience and the cooperation of the patient. Castelijns et al. also stated that MRI is the more sensitive technique for detecting neoplastic cartilage invasion, which may help predict the risk of tumor recurrence. A more recent report by Agada et al. found CT to be about 71% accurate in staging laryngeal cancer pathology (when evaluated against T staging). The otolaryngologist will combine the diagnostic findings with the TNM system of classification in analyzing the information and planning treatment (Stemple et al., 2000, p. 444). Treatment of laryngeal cancer will vary for each individual depending on the site of the cancer, the stage or extent of the cancer, the individual’s age, general health, and personal preferences (American Cancer Society, 2005). A key issue in selection of the most appropriate intervention is implementation of a team approach (Lefebvre et al. 2004). In addition, a vital step in treatment involves the provision of information to individuals with laryngeal cancer, thus enabling an informed treatment decision (Kasperbauer & Tomas, 2004). Kasperbauer and Tomas emphasized that in order to do so, team members must possess knowledge of surgical and nonsurgical options available and must be able to clearly communicated this information to individuals with laryngeal cancer. 6 Intervention strategies include combinations of surgery and or radiation therapy and chemotherapy (American Cancer Society, 2005; Dubravka, Simunjak, Ivkic, & Hedjever, 2003; Dworkin et al., 2003; Lefebvre et al., 2004). Historically, the treatment for advanced-stage laryngeal cancer has been surgery to remove the larynx, however, increasing knowledge of tumor biology has enabled a shift in treatment to methods more conducive to organ preservation (Urba et al., 2006). An essential component of intervention is effective voice restoration which, after removal of the larynx, requires a method of alaryngeal speech (Pou, 2004). Three methods of alaryngeal speech, discussed later in this paper, include electrolarynx, esophageal speech, and tracheoesophageal speech. Organ Preservation: Radiation and Chemotherapy Radiation The discovery of the x-ray by physicist Wilhelm Roentgen in 1895, lead to the development of radiation therapy in the treatment of cancer (Berk, 1995). Roentgen discovered that certain rays, emitted from a vacuum tube, could painlessly penetrate opaque material (such as tissue), causing some redness and blistering which lead to the use of x-rays in the treatment of laryngeal cancer in 1922 (OtoRhino-Laryngology Web, 2003). Today, radiation therapy (also called radiotherapy) utilizes types of high energy waves or particles to shrink or destroy cancerous cells (Stemple et al., 2000, p. 448). External beam radiation therapy is most commonly used in the treatment of early stage (I and II) laryngeal cancer (Korkmaz et al., 2005) or with individuals whose health precludes surgery (American Cancer Society, 2005). External beam radiation is administered from outside the body, as compared to interstitially (within the tissues) (Stemple et al., 2000, p. 447). Korkmaz et al. noted that an advantage of using radiation therapy as the primary treatment in early stage laryngeal cancer, (with surgery used for salvage) is that radiation therapy has been shown to result in superior voice quality as compared to surgical removal of the cancer. 7 Radiation therapy can also work in conjunction with surgery or follow surgery as an adjuvant therapy by itself, or combined with chemotherapy (Dworkin et al., 2003; Urba et al., 2006). Before surgery it can be used to shrink a large tumor and after surgery, it can be used to destroy remaining cancer cells or to treat tumors that grow back (National Cancer Institute, 2003). A typical course of radiation therapy for laryngeal cancer provides one fraction of 2 Gy per day, 5 days per week, for a total of 60-70 Gy (Gy being a unit of absorbed dose of ionizing radiation) (Lefebvre et al., 2004; MerriamWebster, 2002). Individuals who undergo radiation therapy may experience side effects (many of them temporary) which can include: xerostomia (dry mouth), changes in vocal quality (hoarseness), dysphagia, edema of the larynx, sore throat, variations in sense of taste, weight loss, dry skin, skin toxicity, mucositis, radiation burn, and tiredness (American Cancer Society, 2005; Blanco & Chao, 2006). Blanco & Chao found that skin toxicity and mucositis were the most commonly reported side effects and advocated for the provision of supportive care to prevent complications that might result in treatment breaks. Additionally, they stressed that side effects from radiation therapy for any individual will depend on an interplay of patient-related, tumor-related and treatment-factors. Chemotherapy Chemotherapy provides the individual with anticancer drugs, usually taken orally or given intravenously but sometimes given intramuscularly (American Cancer Society, 2005). This method is frequently combined with surgery and /or radiation therapy in treating laryngeal cancers that have metastasized, and is not generally used by itself as a curative treatment (Dworkin et al. 2003; Lefebvre et al., 2004; Oto-Rhino-Laryngology Web, 2003; Robbins, 2005). Chemotherapy is non-specific in its systemic effects and though it does kill cancer cells, the toxicity is significant, damaging normal functioning cells as well (Guadagnolo et al., 2005). Side effects of the drugs used in chemotherapy may 8 include: a temporary loss of appetite, loss of hair, vomiting, and nausea, mouth sores, bleeding or bruising after minor injuries, shortness of breath and tiredness (American Cancer Society, 2005). Antiemetic (antinausea) drugs are given to counteract some of these side effects. The two most commonly used drugs for laryngeal cancer are cisplatin and 5-fluorouracil (Lefebvre et al., 2004; Urba et al., 2006). Methotrexate (an antimetabolite which stops the growth of cancer cells), bleomycin (an antibiotic which destroys cells), and carboplatin (an alkylating agent which stops the growth of cancer cells) are also used in chemotherapy (American Cancer Society, 2005). The American Cancer Society web site includes a section (What’s New in Research) on current research, including a discussion of chemoprevention drugs and treatments aimed at reducing blood supply to tumors, injecting drugs directly into blood vessels that feed tumors and using viruses to change cancer cells into normal cells. Chemotherapy is not usually used alone, but is combined with radiation therapy and frequently utilized in organ preservation protocols for treatment of laryngeal cancer (Blanco & Chao, 2006; Korkmaz et al., 2005; Urba et al., 2006). Radiation and chemotherapy together as a treatment for laryngeal cancer have been shown to decrease the size of tumors more than use of either treatment alone (American Cancer Society, 2005; Hanna et al., 2004). Chemoradiotherapy is now often recommended as the treatment of choice for laryngeal cancer instead of total laryngectomy or other surgeries (Dworkin et al., 2003; Urba et al., 2006). However, a review by Guadagnolo et al. (2005) concluded that toxicity from the various nonsurgical treatments (induction chemotherapy, hyperfractionated radiation therapy, and chemoradiation) remains significantly high. The goal of treatment is survival of the patient, and Dworkin et al. (2003) note that there is debate regarding whether nonsurgical treatment alternatives result in equivalent disease-free survival and quality of life as that obtained with either a partial or total laryngectomy. 9 Surgical Intervention The first successful reported laryngectomy for cancer was performed in 1873 by Dr. Theodore Billroth on a 36 year old man. The man was reported to be hoarse for a three year period and was originally treated through cauterization followed by excision of the tumor leaving the right vocal cord intact. The tumor, however, recurred and the first total laryngectomy was performed with the trachea being sutured to the skin. The patient remained hospitalized for four months and learned to speak using an artificial larynx. He died one year later as a result of tumor recurrence (Oto-Rhino-Laryngology Web, 2003). There are a variety of surgical interventions available to individuals with laryngeal cancer. The type used will depend upon the location of the cancer and its stage. Among the options are total laryngectomy, pharyngo-laryngectomy and near total laryngectomy. In addition, many varieties of partial laryngectomy including hemilaryngectomy, supraglottic laryngectomy, and supracricoid laryngectomy are available. (Casper & Colton, 1998; Weinstein & Laccourreye, 2000). Laser surgery Laser surgery has been used to provide endoscopic resection of supraglottic and glottic cancers as well as cordectomies for early (T1 and T2) glottic cancers (Lefebvre et al. 2004). Lefebvre et al. also reported that voice quality after laser surgery is excellent and the overall cost is less than for open surgery. They noted that laser surgery is an option for salvage treatment. According to a study conducted by Eckel et al. (1998), as cited in Ferlito et al. (2000, p. 460) the survival rate of individuals with glottic cancer receiving laser surgery is approximately 96.7%. A study completed by Ambrosch et al. (1998) found that individuals with supraglotic cancers have a 3-year survival rate of 76% (as cited in Ferlito et al., 2000, p. 460). 10 Total Laryngectomy Total laryngectomy includes the complete removal of the larynx as well as the thyroid and cricoid cartilages, the hyoid bone, two tracheal rings, and the extrinsic strap muscles. This type of surgery is typically indicated for individuals with extensive, bilateral, or severely invasive tumors, for tumors that fail to respond to radiation therapy, and for tumors that recur after conservative treatment (Lefebvre et al., 2004; Stemple et al., 2000, p. 449). In this procedure the airway cannot be maintained, therefore, the severed end of the trachea must be removed from its original position and sutured to an opening in the neck above the notch of the sternum, called the stoma (Pou, 2004). In a large study, Schwartz et al. (2004) determined an overall wound complication (infection) rate of 10% of patients undergoing total laryngectomy. Risk factors for postoperative complications included: preoperative radiation therapy, low albumin, diabetes, and prolonged operative time. A radical neck dissection may be performed if cancer cells have metastasized to the cervical lymph nodes, involving associated veins, nerves and neck muscles (Stemple et al., 2000, p. 449). When tumors extend beyond the larynx and into the pharynx, a pharyngo-laryngectomy may be required. Deschler (2004) noted that though the additional removal of part or all of the adjoining pharynx creates a significant reconstruction challenge, speech rehabilitation will be the same as for individuals undergoing the standard laryngectomy. Near-Total Laryngectomy In a near total laryngectomy, the entire larynx is removed with the exception of one arytenoid and a small band of tissue joining the trachea to the pharynx. A shunt is created from the band of tissue that joins the trachea to the pharynx and allows voice to be generated. Individuals undergoing a near-total laryngectomy typically require a permanent stoma (Kasperbauer, & Thomas, 2004). This procedure is indicated when the tumor does not involve the posterior commissure, one ventricle, and one arytenoid. 11 This treatment method is also used when the vocal folds are severely limited in motion. (Casper & Colton, 1998; Pearson, 1998). Hemi-Vertical Laryngectomy A hemi-vertical or vertical hemilaryngectomy is a form of organ preservation for early glottic cancer where one of the true vocal folds is removed as well as the vocal process of the connecting arytenoids (Rassekh., 2005). The tissue in the interarytenoid area is also eradicated. The area from which the vocal fold is removed is reconstructed with muscle. Although this type of surgery attempts to avoid a permanent stoma, it is possible that some individuals will continue to require the stoma following surgery (Casper & Colton, 1998; Weinstein & Laccourreye, 2000). Rassekh notes that these individuals may experience changes in vocal quality characterized by intermittent hoarseness of up to a few months duration. He warns that vocal cord paralysis may be indicated if the hoarseness changes from intermittent to constant. Supraglottic Laryngectomy A supraglottic laryngectomy involves the removal of structures above the true vocal folds, including the epiglottis, ventricular folds, the upper half of the thyroid cartilage, and the hyoid bone, depending upon where the cancer is located. This surgery is used when the tumor is superior to the anterior commissure (Lefebvre et al., 2004). This type of surgery does not generate a permanent tracheostoma (Kasperbauer & Thomas, 2004). Supracricoid Laryngectomy A supracricoid laryngectomy is another organ preservation surgical technique which does not result in a permanent tracheostoma (Kasperbauer & Thomas, 2004). This type of surgery involves the removal of the entire thyroid cartilage bilaterally, both true vocal folds, and both false vocal folds (Weinstein et al., 2002). Reconstruction following the surgery is completed in one of two ways. Reconstruction may 12 be performed by suturing the cricoid cartilage to the hyoid bone and epiglottis, a technique known as cricohyoidoepiglottopexy (CHEP). However, if the epiglottis is removed during surgery, the reconstruction will involve attaching the cricoid cartilage to the hyoid bone, a procedure known as cricohyoidopexy (CHP) (Bron, Brossard, Monnier, & Pasche, 2000; Weinstein & Laccourreye, 2000). Laccourreye, Laccourreye, El-Sawy, and Weinstein (2000) reported better successes with CHEP reconstruction for early or moderately advanced glottic tumors, as long as the tumor did not extend to the posterior commissure or the cricoid cartilage. In addition, the cancer must not originate on the anterior commissure or in the laryngeal vestibule. Problems associated with this procedure include alterations in vocal quality, laryngeal stenosis, and recurrence of cancerous cells. Lima et al. (2001) indicated that this procedure is useful in treating T2 and T3 glottal cancer. The supracricoid partial laryngectomy with CHP is performed with types of supraglottic and transglottic tumors that do not involve the cricoid cartilage, thyrohyoid membranes, or the hyoid bone. Consequences from this procedure include postoperative bleeding, hematoma, laryngeal stenosis, and the recurrence of cancerous tumors (Brasnu, Hartl, & Laccourreye, 2000). Role of the Speech-Language pathologist ( SLP) Pre-Medical/Surgical Treatment The importance of pre-treatment counseling by the SLP for individuals with head and neck cancer has been advocated by many authors (Gress, 2004; Kasperbauer & Thomas, 2004; Lewin, 2004a; Sparano et al., 2004; Stemple et al., 2000, p. 439). Sparano et al. emphasized that as a member of the voice rehabilitation team, the SLP must have a clear understanding of the pathology and type of surgical resection to be performed and be in agreement with other team members on the postoperative goals for the individual. Preoperative counseling involves counseling the individual on realistic expectations regarding their post-surgical vocal abilities including compensatory strategies for voice and using the 13 new laryngeal configuration (Sparano, et al., 2004). The SLP should also be actively involved in providing the individual with information regarding radiation treatment and chemotherapy, including the side effects (short and long term) of these treatments. When total laryngectomy is planned, the SLP should first discuss the options for rehabilitation with the surgeon and or physician involved in treatment. Some communication options may be contraindicated or not possible due to the type or extent of surgery (Pou, 2004). Options might include tracheoesophageal speech (TE) with prosthesis, esophageal speech, an artificial larynx, augmentative communicative devices, writing, and gestures (Beukelman & Mirenda, 2004; Gress, 2004; Stemple et al., 2000). It is also helpful to provide the individual with published information on the subject in addition to information regarding support groups (Casper & Colton, 1998). This literature is available at no cost through the American Cancer Society, National Cancer Institute, on the internet (e.g. Web Whispers) and through companies that manufacture supplies for individuals with laryngectomy. Pre-operative counseling may also include providing simplistic anatomical pictures that may be used to illustrate the changes that will take place following the procedure. Lewin (2004a) noted that many people do not understand the normal anatomy of the larynx and swallowing, and without preoperative consultation, some individuals may reject surgery due to fears from lack of understanding. It may also be beneficial for the individual to observe other well-rehabilitated individuals using various methods of alaryngeal speech. The SLP should note the individual's vocal quality and speech patterns prior to treatment to determine any implications those factors might have on post-treatment communication options. In addition, the clinician may choose to conduct several informal evaluations, including assessments of cognition, manual agility, visual and auditory abilities, reading and writing skills, and motivation. This 14 information can prove useful in determining a desired method of post-treatment communication (Casper & Colton, 1998). The American Cancer Society (2005) provides some thought provoking questions that individuals faced with laryngeal cancer should consider asking their doctors. SLPs might assist such individuals both pre and post operatively in formulating questions and in discussing the things that concern them. A study conducted by Zeine and Larson, as cited in Koike, Kobayashi, Hirose, and Hara (2002, p. 107) found that 57% (n = 71) of laryngectomized individuals felt that the involvement of the SLP during preoperative counseling was beneficial. This percentage was higher than the ratings of surgeons, nurses, and family doctors in the same study. This statistic is an indicator of the importance of the SLP to a team approach for successful management of laryngeal cancer. Post-Medical/Surgical Treatment The role of the SLP after medical/surgical treatment is one of continued evaluation and support of communication needs including swallowing issues if necessary and alaryngeal voice restoration if applicable. Postoperatively, direct speech intervention begins immediately after surgery (Gress, 2004). Most individuals can use an intraoral type electolarynx immediately following surgery with surgeon's approval (Stemple et al., 2000, p. 477). The focus of the clinician will be to maximize the individual's opportunity to communicate as soon as possible. The clinician should review plans for rehabilitation with the individual, the specifics of which will be determined by the type of treatment the individual receives and the choices that have been made regarding communication options (Casper & Colton, 1998). Kasperbauer and Thomas (2004) advised that near-total laryngectomy individuals should be encouraged to use an oral or neck type of artificial larynx during initial healing. They suggested that this 15 can begin when the individual shows interest and can control saliva and movement of the articulators. With regard to partial laryngeal surgery, Sparano et al. (2004) reiterated the importance of ensuring that the individual has realistic, functional goals for voice production by continuing (postoperatively) to review the concept of functional versus normal voicing. They also emphasized the need for the SLP to collaborate with the oncology team to counter variables which might negatively affect postoperative functional voice outcomes such as medical illness, effects of radiation therapy, and age-related factors. For example, a consideration when working with older individuals with presbycusis, is that decreased hearing acuity will impede their ability to hear even their own voice (which will now have a breathy quality). This may cause them to push harder to increase loudness (Sparano et al., 2004). For individuals undergoing a total laryngectomy, the SLP needs to be knowledgeable about surgical prosthetics. Stemple et al. (2000, 496) listed the different responsibilities of the SLP in assisting individuals with surgical prosthetics including: contributing to evaluation of the individual's suitability for voice prosthesis, sizing and fitting the prosthesis, changing it when needed if it is an indwelling type prosthesis, instructing the individual concerning use, care and cleaning of the prosthesis, helping the individual with optimal use of the prosthesis, and fitting a tracheostoma valve (Schuster et al., 2003). Additionally the SLP should assist in problem solving when leakage through or around the prosthesis occurs or a change in vocal quality is identified. The speech-language pathologist also needs to be aware of and ready to counter problems that affect not only functional voice production but psychosocial aspects that affect communication (Hanna et al., 2004; Stemple et al., 2000, p. 439). These might include worries about health, family and finances, withdrawal from social activities, not answering the phone, and loss of employment (Sparano et al., 2004). Some of these concerns may be directly related to vocal quality and it is important that the SLP continue to educate and work with the individual to optimize voice and communication. Postoperative 16 voice therapy is a crucial part of the recovery process (Zeitels, 2004) and contributes to quality of life (Hanna et al., 2004). Hanna et al (2004) reported differences in quality of life for patients depending on their type of laryngeal cancer treatment. For example, coughing, problems with taste and smell, more need for pain medication, sensory disturbances, and trends toward poorer social functioning (possibly due to disfigurement, disrupted body image, social anxiety, or impaired communication) were reported more often by individuals who had undergone surgery. In contrast, individuals who had undergone chemoradiation reported more problems with dry mouth and sexual concerns. The American Cancer Society (2005) lists some practical solutions to frequent concerns, such as wearing a scarf or turtleneck to ease self-consciousness about the presence of a stoma, and wearing a stoma cover during intimate times to reduce self-consciousness. Avoidance of garlic or spicy foods which can result in odors from the stoma was also suggested. Hanna et al. (2004) found that using an intensive program of voice and speech rehabilitation appeared to lessen perceived speech problems among laryngectomy patients and thus improve quality of life for those individuals. Eadie and Doyle (2005) reported that communication is an extremely important factor in a laryngectomee’s perceived quality of life. Their study highlighted the positive relationship between communication ability and overall well-being, particularly as it relates to the laryngectomee’s willingness to socialize. They found that individuals who were actively involved in laryngectomy support groups had better general health, were less depressed, reported less pain, and were generally better adjusted socially than the “average” alaryngeal speaker. 17 Alaryngeal Speech Artificial Larynx The first artificial larynx was developed in 1859 by a Czech physicist by the name of Johann Cezermak. The tool consisted of a tracheal cannula with a tube and metal reed. The tube was placed in the mouth and the exhaled breath sent the reed into vibration to produce sound. The first recipient of the device was not a laryngectomee, but an 18 year old boy that had undergone a tracheostomy for tracheal stenosis. The artificial larynx was initially used for a laryngectomee in 1873 when the first successful laryngectomy took place (Henslee, n.d.). There are two basic types of artificial larynges, pneumatic and electronic. In the pneumatic type, air from the lungs is diverted through a coupling device held against the stoma. Pulmonary air cuases a reed within the device to vibrate. The air is transmitted through a tube directly into the oral cavity where it is articulated into speech. While the pneumatic type of artificial larynx yields better voice quality than the electronic varieties, it is cumbersome and challenging to use (Kearney, 2004). The more common type of artificial larynx is the electronic variety (Lewin, 2004a). This device generates sound vibrations outside the body, which are then transmitted through the tissues of the neck, cheek, or intra-orally through a tube, so that they can be shaped into speech with the use of the resonating cavities and articulators (Kearney, 2004). Neck placement is preferable but not always possible due to fibrosis from surgery and or radiation. Intra-oral adaptors may be used to bypass the neck and direct the tone into the oral cavity (Eadie, 2003; Kearney, 2004). Esophageal Speech (ES) Esophageal speech is another method of alaryngeal speech. Esophageal speech is generated internally, using the remaining anatomical structures - the cricopharyngeal muscle and the middle and inferior pharyngeal constrictor muscles, collectively called the pharyngeal esophageal (PE) segment. 18 (Stemple et al., 2000, p. 483). This type of speech is produced by the intake of air into the esophagus where it is trapped and then released, causing the PE segment to vibrate. The vibration of the PE segment produces voicing, which is then formed into speech using articulators including the tongue, teeth, and lips. (Eadie, 2003; Kearney, 2004; Lewin, 2004a; Shipley & McAfee, 2004). Individuals using esophageal speech generally use one of two methods or a combination of methods in achieving the air supply needed to produce sound; the inhalation method or the injection method. The inhalation method is a negative pressure approach which draws air into the esophagus by rapid expansion of the thoracic cavity, causing intraesophageal pressure to drop. In order to achieve this, the individual must learn how to relax the PE segment. When this happens, air is passively drawn into the esophagus (Casper & Colton, 1998; Stemple et al., 2000). The injection method is a positive pressure approach which requires the individual to build up high pressure within the oral cavity. This will drive air into the esophagus. There are two phases involved in this method. The tongue must first force air into the mouth and back to the pharynx. Next the pharynx and the back of the tongue drive the air into the esophagus (Casper & Colton, 1998; Kearney, 2004). Lewin (2004a) gave several reasons why esophageal speech is not highly popular. She cited limited familiarity with the method, the time it takes to learn to produce esophageal speech, and a small number of people who actually learn to use this technique for functional communication. Singer (2004) stated that less than one third of laryngectomees are successful at acquiring esophageal speech. Many individuals can provide short phrases of esophageal speech however their speech is not functional for conversation. Stemple et al. (2000, 485) noted that high motivation and work are required to become proficient at this type of speech. Benefits of esophageal speech include a more natural sound than that of an artificial larynx (Shipley & McAfee, 2004). The individual has some control over pitch and loudness and the hands free 19 feature provides greater independence for a more active lifestyle. Esophageal speech does not rely on the use of mechanics, batteries are not needed, nor does this method cost anything (Hocevar-Boltezar & Zargi, 2001). However, esophageal speech is typically lower in volume than tracheoesophageal speech or speech prior to laryngectomy. Individuals who are being considered for ES must have high motivation to put forth the physical and emotional effort needed to produce intelligible speech (Hocevar-Boltezar & Zargi, 2001; Singer, 2004). Medical conditions that may preclude candidacy are listed by Stemple et al. (2000, p. 489) as: post radiation fibrosis, pharyngeal scarring, esophageal stenosis, recurring suture line fistulae, and defects in neural innervation. In addition, some individuals are not candidates for esophageal speech due to lack of relaxation of the PE segment. Tracheoesophageal Puncture (TEP) Another option for individuals who have undergone total laryngectomy is tracheoesophageal voice restoration surgery. The use of a fistula technique for voice restoration was proposed in Billroth's 1873 paper, which described the first successful laryngectomy (Oto-Rhino-Laryngology Web, 2003; Singer, 2004). Many versions of the T-E fistulization followed. However, until the 1980s, either an artificial larynx or esophageal speech continued to be the primary choices for communication. T-E fistulization gained popularity after 1979, when Singer and Blom refined the procedure and prosthesis (ASHA, 2004a). A detailed chronology of the development of tracheoesophageal voice restoration is discussed in Singer (2004). TEP is a surgical voice restoration procedure, also referred to as tracheoesophageal prosthesis and TE surgery (ASHA, 2004a). A one directional, silicon rubber shunt valve is placed in a surgically created T-E fistula (posterior wall of the trachea through the anterior wall of the esophagus) that allows pulmonary air to travel through the prosthesis into the esophagus where esophageal sound is generated 20 through vibration of the pharyngo-esophageal segment (ASHA, 2004a; Singer, 2004; Ten Hallers et al., 2005). The prosthesis also prevents the aspiration of saliva, liquid, and food into the trachea. A flap on the valve opens by positive pressure and closes by elastic recoil during exhalation (Singer, 2004). In order for speech to be produced, the stoma must be covered so that air does not escape at that point, but is forced to continue into the esophagus. This can be accomplished manually or with a tracheostoma valve which will occlude when sufficient air pressure has accumulated (Lewin, 2004a; Stemple et al, 2000, p. 495). Tracheoesophageal voice prostheses can be indwelling or non-indwelling. The non-indwelling prosthesis was developed initially (The Blom-Singer device was referred to as the duckbill voice prosthesis.). A flap valve design improved voicing and allowed the development of a tracheostoma valve, which eliminated the need to occlude the stoma manually. This also improved hygiene and cosmetic appeal (Blom, 2000). Later, an indwelling voice prosthesis was developed which could remain in place for weeks or months. Additionally, heat moisture exchangers were developed which cover the tracheostoma or are incorporated into the devices (Singer, 2004). After a total laryngectomy, the individual is breathing through the stoma instead of the nasal passages. Without a heat moisture exchanger there is no warming, humidifying, or filtering of the air. The heat moisture exchanger protects the airway, maintains a more natural tracheal environment, and decreases mucus production and coughing as the trachea is more protected from drying and cooling (which can cause thick or crusty mucus to form) (Bunting, 2004; InHealth Technologies, 2006; Ten Hallers et al., 2005). Advantages and disadvantages of each type of valve will depend on the individual and his or her specific needs and abilities. An advantage of the non-indwelling prosthesis is that it can be inserted, removed, and cleaned by the patient (InHealth Technologies, 2006). Bunting (2004) noted that the duckbill prosthesis is the least 21 expensive. Advantages for use of the non-indwelling device, cited by Vlantis et al. (2003) included that the devices were older and had been prescribed before the newer models were available. Thus, speech pathologists and surgeons may have had more experience with the older, non-indwelling devices, and because they are less expensive, the non-indwelling devices are the type made available by many institutions (indwelling devices were reported as 100 to 200 percent higher cost than the non-indwelling type). Non-indwelling devices continue to be investigated and improved upon as discussed in a study by Hancock, Houghton, Van As-Brooks, and Coman (2005). Disadvantages noted by Bunting (2004) and Vlantis et al. (2003) included the following. The prosthesis is fixed to the skin with tape and individuals must have the dexterity and cognitive ability to keep up the removal, reinsertion, and cleaning, sometimes daily. Replacement into the tracheoesophogeal fistula is one of the major causes of failure. For example, if the individual does not insert the prosthesis completely into the esophagus, it can lead to a false passage and closure of the distal end of the puncture tract. Advantages of the indwelling prosthesis, cited by Vlantis et al. (2003) included that it can be cleaned in situ and does not have to be removed as frequently. Thus it requires less dexterity, and there is no need for a tag taped to the skin which results in a better seal of the stoma during phonation. Another advantage of the indwelling voice prosthesis is that it maintains the tracheoesophageal tract and does not fall out or become dislodged as can the catheter or stent of the duckbill prosthesis (e.g. with aggressive coughing or while being cleaned) (Bunting, 2004). Vlantis et al. (2003) indicated that over 50 percent of family members who responded to a questionnaire, also preferred the indwelling type of tracheoesophageal voice restoration prosthesis. However for individuals who do not live near a facility or specialist who can remove the device, it becomes an inconvenience to travel long distances (Bunting, 2004; Singer, 2004). Disadvantages of the indwelling prosthesis also include the following: it is more prone to yeast colonization which destroys its function, as microbial colonization can hold the flap valve 22 open, leading to leakage through the prosthesis; there is a need for more frequent replacement of the valve (compounded by the higher cost); and enlargement of the fistula can results in reduced fixation and infections (Bunting, 2004; Ten Hallers et al., 2005). If the individual is receiving radiation treatment the valve will deteriorate in 6 to 12 weeks. Bunting noted that this is a current challenge for manufacturers and designers. One solution has been the Blom-Singer Indwelling Advantage Voice Prosthesis which incorporates silver oxide into the silicon flap valve to preserve the material (InHealth Technologies, 2006). Using the tracheostoma breathing valve is more hygienic than manual stoma occlusion, it allows for hands-free communication, and does not draw unwanted attention to the stoma (Lewin, 2004b). Ten Hallers et al. (2005) discussed development of different types of tracheostoma valves, either fixed to the skin or with an intra-tracheal device. The researchers made recommendations for improvements in how these valves could be attached noting that the method of fixation could result in major problems, and discretion was needed when determining which individuals were suited to a particular valve. Lewin mentioned other differences between devices that the SLP will need to understand when selecting appropriate devices. For example, some prostheses have a small hood projecting from the distal end (in the esophagus) covering the internal valve which may be an advantage for certain anatomical configurations. Some of the tracheostoma breathing valves are spring loaded and some have a small diaphragm that closes with increased pulmonary pressure. Individual patient needs will help determine which features are advantageous and which are disadvantages. InHealth Technologies (2006) lists products for tracheoesophageal puncture and voice restoration in their 2006 ENT Product catalog. Products include a Blom-Singer Humidifilter System which is a heat and moisture exchange, protecting the trachea from drying and cooling This system is used with manual occlusion of the stoma. The Blom-Singer Adjustable Tracheostoma valve II allows hands-free 23 speech as the stoma does not need to be manually blocked. A humidifier cap is available to protect the trachea from drying and cooling. Eadie and Doyle (2005) related the use of a humidifier moisture exchange to ease of breathing and speaking, thereby positively influencing quality of life. They postulated that more efficient breathing would increase general levels of activity and associated independence as well as reduce fatigue. Social functioning and comfort would improve with reduced concerns regarding stoma hygiene and decreased coughing. Additionally, use of the hands-free tracheostoma valve would allow for more normalized communication interactions. The SLP will need to discuss safety issues with individuals who now breathe through a stoma, and with their families (Stemple et al. 2000, p. 468). Medical alert cards or bracelets should be suggested. Individuals should be made aware of safety aides to be used when bathing, showering or around water, to protect the opening of the stoma. The stoma opening would also need to be protected in environments where dust and particles could be inhaled. Stemple et al. related studies reporting that only a small percentage of laryngectomees had difficulty with lifting, however, without the ability to abduct the vocal folds and thus build thoracic air pressure, body functions requiring this extra pressure or exertion may be affected. In some cases, this may preclude the individual’s ability to continue in their previous employment, as lifting heavy objects may now be too difficult. Each alaryngeal speech option has advantages and disadvantages, and as Kearney (2005) points out, SLPs should understand the noninvasive methods (esophageal speech, electronic devices) as well as the surgical methods in order to help make the most informed decision. When deciding candidacy and an adequate method for each individual, factors to consider should include: device/prosthesis design and features of each, matched to patient (and family or caregiver) strengths, cognitive and physical abilities, age, preferences, cost, maintenance, and the outcome of voice quality. The physician and the SLP play an important role in providing adequate voice restoration. ASHA's position statement clearly indicates 24 that the SLP has a primary responsibility in the evaluation and treatment for tracheoesophageal puncture and prosthesis (ASHA 2004b). The success (or failure) of the individual in acquiring an understanding of and the skill to optimize their verbal communication potential is influenced by the efforts of the team of professionals working together with the individual to facilitate his or her voice restoration (Lewin, 2004a). Swallowing Difficulties Dysphagia following Radiation and or Chemotherapy The various types of laryngeal cancer treatment may manifest diverse swallowing issues. Samlan and Webster (2002) reported that radiation therapy may produce short-term and long-term effects on swallowing. The short-term effects may interrupt swallowing competence and safety, and may result in mild to severe dysphagia. The consequences may range from a need to modify the diet to non-oral feeding. Long-term effects that may play a role in dysphagia include edema of the larynx, dryness of the mouth, and reduction of sense of the taste and smell. Chemotherapy alone does not typically result in dysphagia. However, individuals treated with chemotherapy combined with radiation therapy may show an increase in the rate of dysphagia when compared to those treated with radiation therapy alone (Samlan & Webster, 2002). In addition, Salaman and Webster (2002) reported that individuals treated with radiation therapy either before or after surgery are at an increased risk for swallowing dysfunction. Gillespie, Brodsky, Day, Lee, and Martin-Harris (2004) found that individuals treated with chemotherapy in addition to radiation therapy had less swallowing issues than those treated with both surgery and radiation therapy. However, it should be cautioned that organ preservation protocols are newer and the full effects on swallowing function are not completely known. 25 Dysphagia following Surgical Intervention Individuals undergoing total laryngectomy do not typically present with significant dysphagia, though some swallowing difficulties may be present (Stemple et al., 2000, p. 467). Reduced tongue base retraction may slow eating efficiency. Additionally, narrowing in the reconstructed pharynx and or esophagus may create difficulties with solid foods (McConnel, Mendelsohn, & Logemann, as cited in Murray, 1999). Nerve fibers are disrupted in a total laryngectomy resulting in hyper-contracted spasms which stop food from moving down the esophagus. These individuals frequently report taking a longer than normal time to swallow and an increased effort involved in swallowing (Samlan & Webster, 2002). Total laryngectomees with a TEP may also present with swallowing difficulties. Individuals may aspirate through the TE prosthesis if there is a problem with the fit of the prosthesis or the prosthesis itself (Bunting, 2004). Carroll (2002) indicated that aspiration is rare in individuals with total laryngectomy because the trachea and esophagus have been surgically separated. In addition, Ward, Bishop, Frisby, and Stevens (2002) reported that pharyngolaryngectomees may experience swallowing problems similar to those of laryngectomees. They may experience a longer period of non-oral feeding immediately following surgery and a higher rate of complications associated with dysphagia postoperatively. Swallowing problems in those individuals treated with partial laryngectomy procedures may be the result of inadequate protection of the airway due to the structural changes following surgery (Murray, 1999). Supraglottic laryngectomy results in reduced protection of the airway caused by the loss of the ventricular folds and the epiglottis. As a result of deposits in the pharynx, these individuals may aspirate while swallowing or directly thereafter. However, individuals who undergo hemilaryngectomy may have dysphagia for a period following surgery, but often return to functional swallowing after 26 approximately one month unless they undergo radiation treatment and experience effects of radiation (Samlan & Webster, 2002). Samlan and Webster (2002) also indicated that individuals who receive a supracricoid partial laryngectomy (CHEP or CHP) may resume normal diets and maintain efficient swallow mechanisms roughly 75% to 100% of the time. However, the researchers also indicated that there can be problems in the oral and pharyngeal phase of the swallow including reduced tongue movement and reduced laryngeal elevation. Conclusion and Clinical, Social, and Vocational Implications When an individual has been diagnosed with laryngeal cancer and when partial or total laryngectomy is the decided course, the speech-language pathologist, as part of the medical support team for that individual can have a positive impact on that individual and his or her family. The primary goal of laryngeal cancer treatment, whether it be surgical excision and/or chemo-radiation therapy, is to achieve adequate cure of the cancer while retaining maximum physical function. Secondary concerns address communication, swallowing (eating and drinking) and emotional wellbeing. Though not the first concern, voice restoration (if possible), and at least the ability to communicate effectively are vital to the individual's social, psychological and indirectly his or her physical wellbeing. The ability to continue working, socializing and eating are very important to most people. A well trained SLP, knowledgeable and experienced in voice and dysphagia rehabilitation can make a significant difference in the recovery, expectations, and accomplishments of the laryngectomized individual. 27 References Agada, F. O., Nix, P. A., Salvage, D., Stafford, N. D. (2004). Computerised tomography vs. pathological staging of laryngeal cancer: a 6-year completed audit cycle. International Journal of Clinical Practice, 58, 714-6. Altieri, A., Garavello, W., Bosetti, C., Gallus, S., & La Vecchia, C. (2005). Alcohol consumption and risk of laryngeal cancer. Oral Oncology, 41, 956-65. American Cancer Society (2005, June 15). Cancer reference information. Retrieved February 4, 2006 from: http://www.cancer.org/docroot/CRI/content/CRI_2_2_1X_What_are_laryngeal_and_hypoph aryngeal_cancers_23.asp. American Speech-Languge-Hearing Association. (2004a). Evaluation and treatment for tracheoesophageal puncture and prosthesis: Technical report. ASHA Supplement 24, 135-139. American Speech-Languge-Hearing Association. (2004b). Roles and responsibilities of speechlanguge pathologists with respect to evaluation and treatment for tracheoesophageal punctue and prosthesis. ASHA Supplement 24. in press. Berk, R. N. (1995). Eugene W. Caldwell lecture. The American Journal of Roentgenology: past, pesent, and future. American Journal of Roentgenology, 164, 1323-8. Beukelman, D., & Mirenda, P. (2004). Augmentative and Alternative Communication. Baltimore, MD.: Brookes Publishing Co. Blanco, A. I., Chao, C. (2006). Management of radiation-induced head and neck injury. Cancer Treatment and Research, 128, 23-41. Blom, E.D. (2000). Current status of voice restoration following total laryngectomy. Oncology, 14, 915-922. Brasnu, D. F., Hartl, D. M., & Laccourreye, H. (2000). Supracricoid partial laryngectomy with cricohyoidopexy. In G. Weinstein, O. Laccourreye, D. Brasnu, & H. Laccourreye (Eds.), Organ preservation surgery for laryngeal cancer (pp. 127-145). San Diego, CA: Singular Publishing Group, Inc. Bron, L., Brossard, E., Monnier, P., & Pasche, P. (2000). Supracricoid partial laryngectomy with cricohyoidoepiglottopexy and cricohyoidopexy for glottic and supraglottic carcinomas. Laryngoscope, 110, 627-634. Bunting, G. W. (2004). Voice following laryngeal cancer surgery: Troubleshooting common problems after tracheoesophageal voice restoration. Otolaryngologic Clinics of North America, 37, 597-612. Carroll, W. (2002). Laryngectomy faqs. Retrieved on November 3, 2004, from: 28 http://www.larynxlink.com/library/faqs/FAQ10.html. Casper, J. K., & Colton, R. H. (1998). Clinical manual for laryngectomy and head/neck cancer rehabilitation. San Diego, CA: Singular Publishing Group, Inc. Castelijns, J. A., Hermans, R., van den Brekel, M. W. M., Mukherji, S. K. (1998). Imaging of laryngeal cancer. Seminars in Ultrasound CT & MRI, 19, 492-504. Deschler, D. G., Gray, S. T. (2004). Tracheoesophageal speech following laryngopharyngectomy and pharyngeal reconstruction. Otolaryngologic Clinics of North America, 37, 1-14. Dubravka, G., Simunjak, B., Ivkic, M., & Hedjever, M. (2003). Speech and voice analysis after near-total laryngectomy and tracheoesophageal puncture with implantation of Provox 2 prosthesis. Logoped Phonaitr Vocol, 29, 84-86. Dworkin, J. P., Meleca, R. J., Zacharek, M. A., Stachler, R. J., Pasha, R., Abkarian, G. G., et al. (2003). Voice and deglutition functions after the supracricoid and total laryngectomy procedures for advanced stage laryngeal carcinoma. Otolaryngology-Head and Neck Surgery, 129, 311-20. Eadie, T. L. (2003). The ICF: A proposed framework for comprehensive rehabilitation of individuals who use alaryngeal speech. American Journal of Speech-Language Pathology, 12, 189-197. Eadie, T. L., Doyle, P. C. (2005). Quality of life in male tracheoesophageal (TE) speakers. Journal of Rehabilitation Research & Development, 42, 115-124. Eckel, H. E., Schneider, C., Jungehulsing, M., Damm, M., Schroder, U., Vossing, M. (1998). Potential role of transoral laser surgery for larynx carcinoma. Lasers in Surgery and Medicine, 23, 79-86. Ferlito, A., Silver, C. E., Howard, D. J., Laccourreye, O., Rinaldo, A., & Owen, R. (2000). The role of partial laryngeal resection current management of laryngeal cancer: a collective review. Act Otolaryngol, 120, 456-465. Gillespie, B. M., Brodsky, M. B., Day, T. A., Lee, F., & Martin-Harris, B. (2004). Swallowing related quality of life after head and neck cancer treatment. The Laryngoscope, 114(8), 1362-1367. Gress, C. D. (2004). Preoperative evaluation for tracheoesophageal voice restoration. Otolaryngologic Clinics of North America, 37, 519-30. Guadagnolo, B. A., Haddad, R. I., Posner, M. R., Weeks, L., Wirth, L. J., Norris, C. M., Sullivan, C. A., et al. (2005). Organ preservation and treatment toxicity with induction chemotherapy followed by radiation therapy or chemoradiation for advanced laryngeal cancer. American Journal of Clinical Oncology, 28, 371-8. Hancock, K., Houghton, B., Van As-Brooks, C. J., & Coman, W. (2005). First Clinical experience with a new non-indwelling voice prosthesis (Provox NID) for voice rehabilitation after total laryngectomy. Acta Oto-Laryngologia, 125, 981-90. 29 Hanna, E., Sherman, A., Cash, D., Adams, D., Vural, E., Fan, C., et al. (2004). Quality of life for individuals following total laryngectomy vs chemoradiation for laryngeal preservation. Archives of Otolaryngology - Head and Neck Surgery, 130, 875-9. Henslee, J. (n.d.). History of the laryngectomy. Retrieved May 30, 2005 from: www.larynxlink.com/Library/Health/history/htm Hocevar-Boltezar, I., & Zargi, M. (2001). Communication after laryngectomy. Radiology and Oncology, 35, 249-254. InHealth Technologies, (2006). Retrieved February 4, 2006 from: http://www.inhealth.com/indwellingadvantage.htm. Kasperbauer, J. L., & Thomas, J. E. (2004). Voice rehabilitation after near-total laryngectomy. Otolaryngologic Clinics of North America, 37, 1-20. Retrieved October 7, 2004, from the Science Direct database. Kearney, A. (2004). Nontracheoesophageal speech rehabilitation. Otolaryngologic Clinics of North America, 37, 613-25. Koike, M., Kobayashi, N., Hirose, H., & Hara, Y. (2002). Speech rehabilitation after total laryngectomy. Acta Otolaryngol, 547, 107-112. Korkmaz, H., Du, W., Yoo, G., Enamorado, I., Lin, H., Adsay, V., et al. (2005). Prognostic significance of G1 cell-cycle inhibitors in early laryngeal cancer. American Journal of Otolaryngology, 26, 77-82. Laccourreye, O., Laccourreye, H., El-Sawy, M., & Weinstein G. S. (2000). Supracricoid partial laryngectomy with cricohyoidoepiglottopexy. In G. Weinstein, O. Laccourreye, D. Brasnu, & H. Laccourreye (Eds.), Organ preservation surgery for laryngeal cancer (pp. 73-94). San Diego, CA: Singular Publishing Group, Inc. Lee, K. W., Kuo, W. R., Tsai, S. M., Wu, D. C., Wang, W. M., Fang, G. M., et al. (2005). Different impact from betel quid, alcohol and cigarette: risk factors for pharyngeal and laryngeal cancer. International Journal of Cancer, 117, 831-6. Lefebvre, J. L., Coche-Ddequeant, B., Degardin, M., Kara, A., Mallet, Y., Ton Van, J. (2004). Teatment of laryngeal cancer: the permanent challenge. Expert Review Anticancer Therapy, 4, 913-920. Leon, X., Rinaldo, A., Saffiotti, U. & Ferlito, A. (2004). Laryngeal cancer in non- smoking and nondrinking individuals. Acta Otolaryngology, 124, 664-669. Lewin, J. S. (2004a, January 20). Advances in alaryngeal communication and the art of tracheoesophageal (TE) voice restoration. The ASHA Leader, 9, 6-7, 20-21. 30 Lewin, J. S. (2004b). Nonsurgical management of the stoma to maximize tracheoesophageal speech. Otolaryngologic Clinics of North America, 37, 585-96. Lima, R. A., Freitas, E. Q., Kligerman, J., Dias, F. L., Barbosa, M. M., Geroldo, M., Santos, I. C., et al. (2001). Supracricoid laryngectomy with CHEP: Functional results and outcomes. OtolaryngologyHead and Neck Surgery, 124, 258-260. Mc Auliffe, M. J., Ward, E. C., Bassett, L., & Perkins, K. (2000). Functional speech outcomes after laryngectomy and pharyngolaryngectomy. Laryngoscope, 126(6), 705-709. Meleca, R. J., Dworkin, J. P., Kewson, D. T., Stachler, R. J., Hill, S. L. (2003). Functional outcomes following nonsurgical treatment for advanced-stage laryngeal carcinoma. Laryngoscope, 113(4), 720-728. Mendenhall, W. M., Million, R. R., Stringer, S. P., & Cassisi, N. J. (1999). Squamous cell carcinoma of the glottic larynx: a review emphasizing the University of Florida. Southern Medical Journal, 92(4), 385-393. Merriam-Webster (2002). Merriam-Webster’s Medical Desk Dictionary (Revised ed.). Springfield, MA.: Merriam-Webster, Inc. Murray, J. (1999). Manual of Dysphagia Assessment in Adults. San Diego, CA: Singular Publishing Group, Inc. National Cancer Institute (2003, May 5). What you need to know about cancer of the larynx. Retrieved February 3, 2006 from: http://www.nci.nih.gov/cancertopics/wyntk/larynx/page3. Oto-Rhino-Laryngology Web (2003). Laryngectomy History. Retrieved February, 4, 2006 from: http://www.orl.nl/Voice_Rehabilitation/History/history.html. Pearson, B. W. (1998). Surgical options in laryngeal cancer. In K. Robbins & T. Murry (Eds.). Head and Neck Cancer (pp. 37-44). San Diego, CA: Singular Publishing Group, Inc. Pou, A. M. (2004). Tracheoesophageal voice restoration with total laryngectomy. Otolaryngeologic Clinics of North America, 37, 1-12. Qadeer, M. A., Colabianchi, N., & Vaezi, M. F. (2005). Is GERD a risk factor for laryngeal cancer? Laryngoscope, 115, 486-491. Rassekh, C. H. (2005). Conservation laryngeal surgery, vertical hemilaryngectomy. EMedicine. Retrieved February 4, 2006 from: http://www.emedicine.com/ent/topic692.htm. Robbins, K. T. (2005). Selecting from the menu of treatment options for locally advanced laryngeal cancer. Archives of Otolaryngology-Head and Neck Surgery, 131, 819. 31 Roychowdhury, S., Roychowdhury, G., & Sen, U. (2005). Assessment of awareness level on tobacco and smoking habits as risk factors for cancer among lung and laryngeal cnacer patients in Kolkata – a case control study. Asian pacific Journal of Cancer prevention, 6, 332-6. Samlan, R. A., & Webster, K. T. (2002). Swallowing and speech therapy after definitive treatment for laryngeal cancer. Otolaryngologic Clinics of North America, 35, 15-16. San Diego Center for Voice and Swallowing Disorders. (2004). Laryngeal cancer. Retrieved October 02, 2004, from www.sandigovoice.org/larynxca.html Schuster, M., Lohscheller, J., Kummer, P., Hoppe, U., Eysholdt, U., & Rosanoweski, F. (2003). Quality of life in laryngectomees after prosthetic voice restoration. Folia Phoniatrica et Logopaedica, 55, 211-219. Schwartz, S. R., Yueh, B., Maynard, C., Daley, J., Henderson, W., & Khuri, S. F. (2004). Predictors of wound complications after laryngectomy: A study of over 2000 patients. Otolaryngology-Head and Neck Surgery, 131, 61-8. Shipley, K. G., & McAfee, J. G. (2004). Assessment in speech-language pathology: A resource manual. Clifton Park, NY: Delmar Learning. Singer, M. I. (2004). The development of successful tracheoesophageal voice restoration. Otolaryngologic Clinics of North America, 37, 507-17. Sparano, A., Ruiz, C., & Weinstein, G. S. (2004). Voice rehabilitation after external partial laryngeal surgery. Otolaryngologic Clinics of North America 37, 637-53. Stemple, J., Glaze, L., & Klaben, B. (2000). Clinical Voice Pathology (3rd edition). San Diego, CA: Singular Publishers. Ten Hallers, E.J., Marres, H. A., Rakhorst, G., Hagen, R., Staffieri, A., Van Der Laan, B. F., et al. (2005). Difficulties in the fixation of prostheses for voice rehabilitation after laryngectomy. Acta Oto- Laryngologica, 125, 804-13. Thekdi, A. A., & Ferris, R. L. (2002). Diagnostic assessment of laryngeal cancer. Otolaryngologic Clinics of North America, 35, 1-15. Urba, S., Wolf, G., Eisbruch, A., Worden, F., Lee, J., Bradford, C., et al. (2006). Single-cycle induction chemotherapy selects patients with advanced laryngeal cancer for combined chemoradiation: a new treatment paradigm. Journal of Clinical Oncology, 24, 593-598. Vlantis, A. C., Gregor, R. T., Elliot, H., & Oudes, M. (2003). Conversion from a non-indwelling to a Provox 2 indwelling voice prosthesis for speech rehabilitation: Comparison of voice quality and patient preference. The Journal of Laryngology & Otology, 117, 815-820. Ward, E. C., Bishop, B., Frisby, J., & Stevens, M. (2002). Swallowing outcomes 32 following laryngectomy and pharyngolaryngectomy. Archives of Otolaryngology- Head Neck Surgery, 128, 181-186. Weinstein, G. S., & Laccourreye, O. (2000). Organ preservation surgery of the larynx: A new paradigm. In G. Weinstein, O. Laccourreye, D. Brasnu, & H. Laccourreye (Eds.), Organ Preservation Surgery for Laryngeal Cancer (pp. 1-11). San Diego, CA: Singular Publishing Group, Inc. Weinstein, G. S., Laccourreye, O., Ruiz, C., Dooley, P., Chalian, A., Mirzan, N. (2002). Larynx preservation with supracricoid partial laryngectomy with cricohyoidoepiglottopexy. Annals of Otology, Rhinology & Laryngology, 111, 1-7. Zeitels, S. M. (2004). Optimizing voice after endoscopic partial laryngectomy. Otolaryngologic Clinics of North America, 37, 627-36.