Bohr-Rutherford Model of the Atom
advertisement

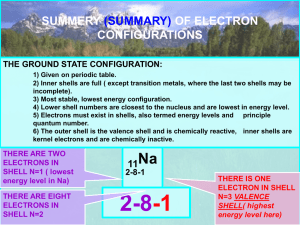
SNC 1D1 Chemistry: Models of Atomic Structure Bohr-Rutherford Model of the Atom Bohr-Rutherford Diagrams 1st shell 2nd shell 3rd shell 4th shell Nucleus Electron shells ( Nucleus ( Electrons ( ) ) ) Steps to draw Bohr-Rutherford Diagrams: 1. Write out a PEN statement: number of protons, electrons, neutrons 2. Draw a circle for the nucleus: write down the number of protons and neutrons in the nucleus. 3. Draw the first electron shell: draw a dot for each electron (at 12 and 6) – stop once you’ve placed all electrons 4. Draw the second electron shell: draw a dot for each electron (at 12, 3, 6, 9); now, pair them up – stop once you’ve placed all electrons 5. Repeat for the third and fourth electron shells. **DO NOT MOVE ON TO THE NEXT ELECTRON SHELL UNTIL YOU HAVE FILLED THE ONE BEFORE IT**