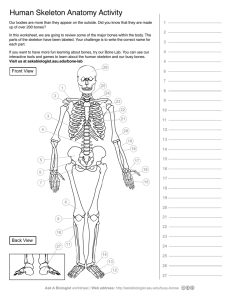
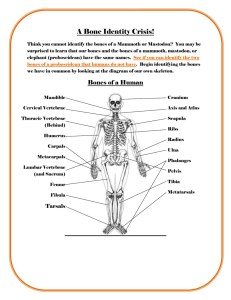
Chordate diversity - Idaho State University
advertisement

Comparative Vertebrate Anatomy Fall 2003 BIOS 314 lecture: 11:00-12:00 Monday and Wednesday lab: 9:00-11:50 or 1:00-4:00 Thurs Instructor: Curt Anderson, Ph.D. Office: LS 331 Research lab: LS 330 Phone: 282-5813 e-mail: andecurt@isu.edu homepage: www.isu.edu/~andecurt Office hours: TBA Objectives To explore the phylogenetic underpinnings of vertebrates and to develop an appreciation for the comparative approach for understanding structure and functional design. This course will survey the gross structure of most major vertebrate groups with a focus on the functional, evolutionary, developmental and physiological mechanisms that influence the design of organisms. Required Texts Vertebrates; comparative anatomy, function, evolution. 2002. Kardong. 3rd edition (second edition is okay, too, but the 3rd is better), Harcourt College Publishers. ISBN: 0072909560 (used copies may be available. try Half.com or Amazon.com) Course Policies Students are expected to attend all lecture and laboratory sessions. If you have extenuating circumstances and must miss, notify me ahead of time in order to schedule a make up exam (make up exams are generally more difficult). Unexcused absences will result in a zero for that quiz/examination. Quizzes will be given weekly in lab and missed quizzes can not be made up. Throughout the semester, the correct answer to 'will this be on the test?' is yes. Unavoidably, this course is a relatively expensive one. Please see the laboratory page for a list of required equipment in addition to the texts. ISU Official Policy on Academic Integrity Academic integrity is expected of all individuals in academe. Behavior beyond reproach must be the norm. Academic dishonesty in any form is unacceptable. Academic dishonesty includes, but is not limited to, cheating and plagiarism. CHEATING is defined as the act of using or attempting to use, in examination(s) or other academic work, material, information, or study aids which are not permitted by the instructor. PLAGIARISM is defined as representing another person’s words, ideas, data or work as one’s own. Plagiarism includes, but is not limited to, the exact duplication of another’s work and the incorporation of a substantial or essential portion thereof without appropriate citation. Other examples of plagiarism are the acts of appropriating the creative works in such fields as art, music and technology, or portions thereof, and presenting them as one’s own. A note on dissections: EVERY student MUST participate in the dissection exercises. The specimens we will be viewing come from supply companies that are required by law to ensure that the animals are euthanized in a safe and humane manner. These animals were euthanized for our educational benefit and the proper level of professionalism will be maintained. If you have ethical or moral objections to dissecting animals, you should drop the course (BIOS 324 is the alternate course to fulfill the requirements of the zoology major). Writing assignment The purpose of the assignment is to: supplement the material you are learning in lecture and laboratory force you to discover the primary literature (and where it is in the library) pursue a topic in comparative anatomy that is of interest to you specific requirements: Choose a topic of interest to you and relevant to the topic of comparative anatomy/functional morphology. Using any literature sources you choose (but at least 5 MUST be from the primary literature!), you will summarize the appropriate research in an 8-10 page, double-spaced report. As budding scientists, you will almost certainly be writing many more such reports in your future. As such, writing, grammar and spelling in addition to content will be taken into account when considering your grade for the report. Here are some potential topics to give you an idea of what is expected: allometry of the vertebrate brain theories on the evolution of flight functional anatomy and evolution of the lungs of flying vertebrates moving on land: optimizing for minimum cost optimality in the design of bony elements Grading Procedures The University has instituted a grading policy that includes the use of a + and - in addition to the letter grade. The new grading averages will be as follows: A AB+ B BC+ C CD+ D DF (93.0 - 100%) (89.5 - 92.9%) (87.0 - 89.4%) (83.0 - 86.9%) (79.5 - 82.9%) (77.0 - 79.4%) (73.0 - 76.9%) (69.5 - 72.9%) (67.0 - 69.4%) (63.0 - 66.9%) (59.5 - 62.9%) (< 59.5%) Your course grade will be based roughly on: 3 lecture exams 100 points each 2 lab exams 100 points each lab quizzes 50 points total written project 100 points (25 points outline; 75 points report) total 650 points Tentative Lecture schedule Lecture # 01 Mo 02 We Mo 03 We 2 04 Mo 05 We 06 Mo 07 We 08 Mo 09 We Mo 10 We 11 Mo 12 We 13 Mo 14 We 15 Mo 16 We 17 Mo 18 We Mo 19 We 20 Mo 21 We 22 Mo 23 We Mo We 24 Mo 25 We 26 Mo 27 We Fr Date Aug 25 Aug 27 Sep 01 Sep 03 Topic Introduction/expectations Evolution and Systematics I no class – Labor Day Systematics/Phylogeny of Chordates Sep 08 Sep 10 Sep 15 Sep 17 Sep 22 Sep 24 Sep 29 Oct 01 Oct 06 Oct 08 Oct 13 Oct 15 Oct 20 Oct 22 Oct 27 Oct 29 Nov 03 Nov 05 Nov 10 Nov 12 Nov 17 Nov 19 Nov 24 Nov 26 Dec 01 Dec 03 Dec 08 Dec 10 Dec 19 Phylogeny of Chordates II Chapt 3 Phylogeny of VertebratesII Phylogeny of Vertebrates II Biological Design Chapt 4 Structural Materials Integument Chapt 6 Exam I /writing assignment outline and topic due Muscles The Skull Chapt 7 The Vertebrate Axis Chapt 8 The Vertebrate Skeleton Vertebrate Locomotion -- girdles and limbs pp. 142-144; 339-348 Vertebrate Locomotion -- swimming and flying pp. 144-146; 347-354 Respiratory I Chapt 11 Respiratory II Circulation I Chapt 12 Exam II /writing assignment due Circulation II Digestion and Feeding I Chapt 13 Digestion and Feeding II Urogenital I Chapt 14 Urogenital II comparative avian muscle study -- no class comparative avian muscle study -- no class Nervous System: organization Chapt 16 Nervous System: organization II Nervous System: sensory I Chapt 17 Nervous System: sensory II Final (7:30am - 9:30am) pp. 20-27 I Chapt BIOS 314L/514L thursdays 9-12; 1-4pm Instructor: C. Anderson, PhD Office: Bios 331 Phone: 282-5813 e-mail: andecurt@isu.edu required text: Comparative Vertebrate Anatomy 2002. Kardong and Zalisko. 3rd edition (much better than 2nd edition) ISBN: 0072909579 (used copies may be available. try Half.com or Amazon.com) required equipment: large scissors (5-5.5") small, straight scissors fine tipped forceps large forceps metal scalpel handle (avoid plastic handles) blunt probe (Huber probe) protective gloves (an entire box/student is recommended) lab coat (optional, but recommended) Many of the dissections will be done in pairs, so you could potentially get by if you want to purchase one set of dissecting tools/pair. However, I'd recommend each owning one. The kits at the bookstore are cheap and of not much quality. I will have kits made available for you to purchase through the ISU Biology Department stockroom The dissection tools will be required by lab 06. You may also purchase a box of gloves from Medical Mart or the Biological Sciences stockroom. Tentative Laboratory schedule Week # 01 02 03 04 05 06 07 08 09 10 11 12 13 14 15 16 Date Aug 28 Sep 04 Sep 11 Sep 18 Sep 25 Oct 02 Oct 09 Oct 16 Oct 23 Oct 30 Nov 06 Nov 13 Nov 20 Nov 27 Dec 04 Dec 11 Topic No labs this week classification/cladistics the vertebrate body/vertebrate skull post-cranial skeleton I post-cranial skeleton II musculature I musculature II PRACTICAL #1 general internal anatomy circulation I circulation II urogenital system nervous system I thanksgiving break--no lab nervous system II and review PRACTICAL #2 BIOS 314L Laboratory 1 Chordate diversity goals: To become familiar with the extant groups of the phylum chordata and the process of creating a cladogram. What you need to know for the exam: 1. You must be able to identify the taxonomic position of any chordate to the level of the labels provided in lab. 2. You should know two or three characteristics of each group. 3. You should be able to reconstruct the cladogram of chordates provided. 4. You should be able to construct a cladogram from a data sheet. See attached cladograms for what material you should know. Note that I have erased some of the information from the figures in the text. Below lists a brief description of each group. The bold words are words you will be responsible for. Refer to this as you peruse the specimens. UROCHORDATA (tunicates) This interesting group is represented here by several types of tunicates. Although this is not apparent from the anatomy of the adults, urochordates are clearly allied with the rest of the chordates. The larvae of these animals have notochords and segmented muscles in addition to the pharyngeal "gill" slits seen in the hemichordates (Fig. 2.5). The notochord and muscle are used for swimming and are lost when larvae metamorphose into a sedentary adults. Be sure to examine the slide of a tunicate larvae . CEPHALOCHORDATA Commonly called lancelets or Amphioxus, Branchiostoma are small marine chordates which can be found in shallow coastal regions of our oceans. They are capable of swimming but spend the majority of their time sitting tail down in the sediment and filter feeding. They possess many pharyngeal slits and a distinct notochord. They also have a dorsal hollow nerve cord and segmented body muscles (myomeres) (Fig. 2.6, 2.7). Water is flushed into the pharynx with cilia in the oral cavity. Food is trapped as the water flows out the pharyngeal slits and is passed into the gut. MYXINOIDEA (Myxiniformes) The hagfish (Fig. 3.2) have traditionally been lumped with lampreys and the extinct ostrachoderms in the class Agnatha because they all lack jaws. However, recent work indicates that this may not be a natural group. As the evidence is still inconclusive, we will still refer hagfish and lampreys to the Class Agnatha but will relegate them to distinct groups under this heading. Hagfish are scavengers of the deep sea. They burrow right through carcasses, using a rasping "tongue" to shear off tissue. They have amazing mucus glands in their skin and can turn a bucket of water into a bucket of jello-like slime in seconds. They only do this when disturbed and it is thought to be a means of evading predators. PETROMYZONTIDA (Petromyzontiformes) The lampreys (Fig. 3.6, 3.8 are found in both marine and fresh water. They have complex life histories that involve a larval stage. The larvae, or amocoetes, superficially resemble Branchiostoma but differ in important ways. The amocoete (Fig. 3.10) has relatively few pharyngeal slits, gills, eyes, and it pumps water into the pharynx using muscular contractions rather than cilia. Some adult lampreys continue to filter feed but others are predacious, like the sea lamprey, sucking body fluids out of fish which they attach themseveles to with a specialized disk-shaped mouth. They have a single, dorso-medial nostril. Chondrichthyes -- ELASMOBRANCHII This group is made up of the sharks and rays. These animals have true jaws which are not attached to the braincase, and paired fins. They have a spiracle, paired nostrils and well developed sensory systems including a lateral line and electrosensory pits on the head. They have a secondarily derived cartilagenous skeleton (that is, their ancestors had bony skeletons) and no skull. Skates and rays are primarily bottom dwellers and have enormous pectoral fins that give them a disc like appearance. Most sharks have stream-lined bodies that help make them effecient swimmers. Male sharks and rays have "claspers" by the cloaca that allow them to internally fertilize females, which either produce large yolky eggs or are ovoviviporous (hold eggs internally and give birth to live young). Notice the characteristic heterocercal (asymetrical) caudal fin and the lack of visible scales. Chondrichthyes -- HOLOCEPHALI The ratfish or chimaeras are primarily deep sea animals. They feed on mulluscs and have large tooth plates on the roof of the mouth that are fused with the braincase (unlike the upper jaw of sharks and rays). Like the elasmobranchii, they have cartilagenous skeletons, well developed sensory systems, no scales on the skin and paired fins. They differ from sharks in having a single external gill opening and no spiracle. Actinoptyerygii -- POLYPTERIFORMES This group of actinopterigian fish is from Africa and is composed of two extant genera. You can examine a preserved Polypterus here. Notice the all dorsal fins,the single external gill opening and its distinctly scaly skin. This group differs from other rayfinned fish in having fleshy lobes extending onto the fins. They have well formed lungs and can live in stagnant pools by breathing air. These animals have bony skeletons. Actinoptyerygii -- ACIPENSERIFORMES (Chondrostei) Like the members of the class Chondrichthyes, these fish have secondarily cartilagenous skeletons,heterocercal caudal fins and lack scales. The sturgeon does have some bone plates on its skin. The fins of these animals are supported by rays (as opposed to the lobes seen in Polypterus) The paddlefish (Polyodon), with its huge, paddle-shaped rostrum is found only in the Mississippi drainage while sturgeons are found throughout the Northern Hemisphere. Polydon is a filter feeder and sturgeons primarily are benthic (bottom) feeders. Actinoptyerygii -- LEPISOSTEIFORMES The gars are found only in North America. They have ganoid scales and long, thin jaws, well armed with teeth used in catching other fish for food. They have a bony skeleton and ray fins. Actinoptyerygii -- BOWFINS (Amia) The Bowfin (Amia) is the last surviving member of this group. It is found in central and southern North America. Amia lacks the ganoid type scales of its ancestors and has a more complex feeding mechanism as well. This involves a rotating maxillary bone that occludes the side of the mouth during suction feeding. TELEOSTEI The teleosts are by far the most diverse group of vertebrates. We have a tiny fraction of this group out for your examination but this should give some idea of how incredibly diverse this group is. The feeding mechanism of teleosts is even more complex than that of Amia and allows significant protrusion of the jaws to catch prey. Many forms have a second set of fully functional jaws in the throat called pharygeal jaws. They lack lungs but have swim bladders used for buoyancy and some have evolved new breathing organs. These animals are found in the deepest parts of the oceans, high in mountain lakes and in ponds in the middle of deserts. ACTINISTIA -- Coelocanths These sarcopterigians were thought to have gone extinct during the Cretaceous until Latimeria was discovered in 1939. This "living fossil" lives around the Comoro Islands in the Indian Ocean. It has no lungs, no internal nostrils and a primarily cartilagenous skeleton. However, these are apparently adaptions for dwelling in deep water and characteristics such as fleshy fins and features of the skull indicate that it is closely related to the tetrapods. These animals are ovoviviparous. DIPNOI -- lungfishes There are three genera left in this formerly diverse group. Neoceratodus is found in Australia, Lepidosiren in South America and the animal on display, Protopterus from Africa. They all have well formed lungs and Protopterus and Lepidosiren can survive long periods (18+ months) without any water. They fill their lung by pumping the buccal floor and forcing air inside. These animals have fleshy fins and cosmoid scales. LISSAMPHIBIA These highly derived animals are extremely different from the amphibians that gave rise to reptiles. There are three extant orders. The Gymnophiona (Caecilians) are tropical, burrowing animals that have secondarily become limbless. They are found throughout the rainforests of the world. The Caudata (salamanders) are found throughout the Northern Hemisphere and in South America. Most have four legs but they are greatly reduced in Amphiuma and only the pectoral(front) limbs are present in Siren. One large group of salamanders, the Plethodontids, have lost their lungs and breath through the skin. The Anura (frogs) are found almost everywere. They have a reduced number of vertebrae, no tail, and long hind legs for jumping. All of these groups had a larval stage primitively but direct developement, ovoviviparity and viviparity have evolved in different lineages. The lissamphibia can generally be defined as having four legs, a larval stage, lungs and moist skin. TESTUDINES The tortoises and turtles represent anapsid (no temporal fenestra) reptiles. They have a shell and a pelvic girdle that is inside the ribcage, unlike all other tetrapods and they have no teeth. These animals range from being completely aquatic (i.e sea turtles) to completely terrestrial (the dessert tortoise) and have a wide variety of feeding habits. They lay amniotic eggs with leathery shells. Lepidosauria -- SQUAMATA This group, decended from diapsid reptiles, is comprised of lizards, snakes and amphisbaenians. These animals have tough, impervious skin, lay amniotic eggs (may have secondarily evolved ovoviviparity) and have teeth. Snakes, most amphisbaenians and many lizards are secondarily legless. Amphisbaenians have distinictinve annuli (rings of flesh). Snakes and legless lizards can be externally distinguished because snakes never have eyelids, ear openings or autonomous tails. Lepidosauria -- RHYNCHOCEPHALIA (tuatara) The only living member of the "beak heads" is the tuatara (Sphenodon) of New Zealand. This strange beast maintains a much lower temperature than other reptiles, has a large pineal eye and may live to be fifty years old. CROCODILIA This group is represented by Alligators, Caiman, Crocodiles and the Gavial of India. These superficially lizard-like beasts live throughout the tropics and subtropics. They have osteoderms in the skin and teeth set in sockets. Some species build nests, and help the young hatch out of their eggs and guard them for some time after birth. AVES This group is derived from a group of dinosaurs and primitively had teeth, a tail, and claws on the wings. They have feathers, no teeth, usually provide extensive parental care to their offspring and the ability to fly. The distinctive keeled breast bone and reduced digits on the forelimbs are an easy way to identify a bird skeleton even without the head. They have a unique and highly efficient unidirectional respiratory tract that allows them to fly at great heights. Even though they have no teeth to chew with, they have a gizzard before the stomach in which they grind up food with pebbles. MAMMALIA These animals are derived from synapsid reptiles. They have fur and mammary glands with which they feed their young. They are endotherms (producing their own heat) and maintain high internal temperatures when active. Three distinct groups are extant. The monotremata are represented by the platypus and echidna of Australia and New Guinae. These animals still lay eggs and do not have nipples, the young licking a patch of skin over the mammary gland. The remaining Theria can be subdivided into two groups. The metatheria, or marsupials, are most common in Australia but several species of opposum range in North and South America. These animals give birth after a short gestation period and the neonate completes development in a pouch on the mother. The eutherians, or placental mammals, have an extended gestation period, during which the young gets nutrients and oxygen via a placenta. An introduction to phylogenetics and systematics Cladistics is a method of analyzing the evolutionary relationships between groups to construct their family tree. It has been around for almost fifty years, but has really become popular in the past two decades. The principle behind it is that organisms should be classified according to their evolutionary relationships, and that the way to discover these relationships is to analyze what are called primitive and derived characters. Primitive characters are those attributes of a plant or animal that all members of the group possess. Having four legs is primitive for mammals; they inherited this characteristic from their common ancestor (a proto-mammal or mammal-like reptile). Primitive characters are of no use in analyzing the relationship of organisms within a particular group. Were you to try to construct a family tree for all mammals, it is not helpful to note that they all have four legs. They do, but it doesn't help you in determining who is related to whom. In the jargon of cladistics, primitive characters are called plesiomorphic. Primitive characters shared by all members of the group in question are called symplesiomorphic. (Note: there is no such thing as a primitive organism, only an organism that retains primitive characteristics!) Derived characters are advanced traits which only appear in some members of the group. Cladistics is based on the assumption that the appearance of shared derived characters (synapomorphies) gives clues to evolutionary relationships. A derived character, for example, for some mammals might be loss of the tail, which occurs in the great apes and man. It is assumed that loss of the tail occurred only once, in the common ancestor of apes and man, and that none of us has one because we inherited that trait from our common ancestor. Thus if mammals are separated into groups which do and which don't have a tail, shown by a fork on the evolutionary diagram (cladogram), this represents the point at which a new species evolved which didn't have a tail. Man and the great apes are assumed to have descended from this species. It is important to note that the designation of primitive and derived characters has meaning only when related to the group under study. A character that is derived relative to one group may be primitive for a less inclusive group. The occurrence of fur is a derived character if one is studying all tetrapods (four-footed vertebrates), and serves to distinguish mammals from their ancestors, the reptiles. However, it is a primitive character for the group consisting only of all mammals, and is not useful for determining relationships within the Mammalia. The premise behind a cladistic analysis is that by examining suites of primitive and derived characters, diagrams can be drawn which illuminate the evolutionary relationships between the groups. Branching points (nodes) on the diagram are generated every time a derived character (or group of them) is identified which one group possesses and another does not. The two groups on alternate sides of a node are called sister-groups. By analyzing enough different characters or traits, eventually, it is hoped, a true picture of the family tree can be generated. The goal is to create a diagram where all members of the analysis are descended from a single, common ancestral species, and for which all descendant species are included. This is called a monophyletic group. If all members of a group are not descended from a single common ancestor, the group is termed polyphyletic. If the group doesn't contain every descendant of that common ancestor, it is called paraphyletic. An example of a paraphyletic group is the reptiles. The Class Reptilia in its traditional sense is a useful concept, but it doesn't contain all the descendants of a common ancestor, because mammals and birds are generally placed in their own classes. There are some factors which complicate a cladistic analysis. One is convergent evolution. Bipedality was a characteristic of man. But if your analysis included all groups of mammals, you would probably note that kangaroos are also bipedal. Does this mean that they should be considered closer relatives of man than are the great apes? This problem is handled by including as many different characters as possible in a cladistic analysis. (parts of the above modified from 'What is Cladistics' by Lynne M. Clos) The best way to begin to set up a cladogram is to define a data matrix. Using mammals as an example, we can create the following: 1. 2. 3. 4. character # 1 2 3 4 fish reptiles monkeys apes man 0 1 1 1 1 0 0 1 1 1 0 0 0 1 1 0 0 0 0 1 Has four legs (no = 0; yes = 1) Has fur (no = 0; yes = 1) Lacks a tail (no = 0; yes = 1 Walks bipedally (no = 0; yes = 1) Most data matrices are much, much more complicated (more defined characters allows greater resolution). These matrices are then run through various computer software programs. However, in our example, we could easily create the following cladogram: fish reptiles monkeys apes man synapomorphies What do each of the nodes represent? What is the value of using an outgroup (fish) in your cladogram? Where would you put snakes? Why? 5. Now, lets try creating a cladogram with your own data matrix: Define your character states and place the “animal” on the correct line according to the acquired characteristics you defined. Car Horse-drawn Cart Plane Space Shuttle Synapomorphies 2. Create a data matrix and fill in the following cladogram. Animals Bird Fish Frog Lizard Mouse Shark Snake Whale Characteristics notochord vertebrae feathers tetrapod hair mammary glands cleidoic Egg Data matrix: Bird Fish Frog Lizard Mouse Shark Snake Whale 3. Create a cladogram given the following data matrix: legs stapes diapsid skull cleidoic egg amnion zebra fish 0 0 0 0 0 frog 1 1 0 0 0 iguana 1 1 1 1 1 snake 0 1 1 1 1 4. Now try your hand creating a cladogram using the numbered mammal skulls on the back table….. 5. Questions: what is cladistics? what is a synapomorphy? what does primitive mean? define homology and homoplasy and give an example of each what is meant by a monophyletic group? paraphyletic? give examples of each LAB 2 – The Cranial Skeleton INTRODUCTION Connective tissues include bone, cartilage, fibrous connective tissue, adipose tissue, and blood. The extracellular matrix of connective tissues determine the physical properties of the tissue, and hence its functional role. Cartilage and bone are specialized connective tissues that constitute the skeletal system proper. There are numerous ways to classify bones. One method is to look at the embryonic origins of bone. Endochondral bone is the formation of a cartilage model of the future bone from mesenchyme (generalized embryonic tissue) and the subsequent replacement of this cartilage model by bone tissue. Dermal bone forms directly from mesenchyme without a cartilage precurser. Sesamoid bones form within tendons and are not preceded by a cartilage model. The patella bone of the knee is one example. We will spend much more time in lecture discussing the development and structure of bone and cartilage. In this lab, however, we will begin to explore the vertebrate skeletal system. There are numerous ways to collectively group skeletal elements together. One method, used by your lab manual is to identify an integumentary skeleton, composed of the dermal bone. These are superficial bones such as scales and teeth. We then can identify an endoskeleton. Within the endoskeleton there is a somatic skeleton composed of a axial skeleton (those bones associated with the long axis of the body) and an appendicular skeleton (primarily those bones associated with paired appendages). The other part of the endoskeleton is the visceral skeleton, those structures associated with the jaws, tongue, and gills. In this lab we are going to focus on the ‘cranial’ skeleton; those structures associated with the skull. Thus, we will view portions of the axial as well as visceral skeleton. What follows is a description of parts of your lab manual you should read and a list of terms you should be able to identify on the representative skulls. In lecture, we will be focusing on the function of many of these bones, but for now, lets focusing on identifying them HANDLING OF SKULLS - WARNINGS THOU SHALL NOT TOUCH SKULLS WITH PEN or PENCIL TIPS! Use a blunt probe or dissecting probe. Dried skulls of small fishes are very fragile, especially if the pectoral & pelvic girdles are attached. HANDLE WITH CARE. If a skull was found in a box, return it to the correct box. DO NOT pile one skull on top of another skull. DO NOT balance a skull on the top of a box that is too small for the skull. GENERALLY, Be able to IDENTIFY the following: Taxon of each skull. Names of bones or cartilages on list for each specimen. Names of cartilaginous or bony structures as noted on list for each specimen. TAXON CHONDRICHTHYES Read: Lab Manual pg. 65-80 Dogfish/Squalus (figs. 5.28,5.29 olfactory/nasal capsule (often broken), orbital capsule (orbit) otic capsule occipital region occipital condyle. basal plate TAXON ACTINOPTYERGII Bowfin skull (figure 5.30) Premaxilla Maxilla Nasal Parietal Supratemporal post temporal opercular Postinfraorbitals post orbital sub orbital TAXA LISSAMPHIBIA Read p. 71 Anuran (Bullfrog) skull (handout) Premaxilla nasal Frontoparietal Maxilla Pterygoid Orbit Occipital condyle Foramen magnum TAXON TESTUDINES Turtle skull (fig. 5.33) Premaxilla Maxilla Orbit Frontal Postorbital Parietal Supraoccipital Foramen magnum, Dentary Alligator Skulls (fig. 5.32) Nares Premaxilla Maxilla Prefrontal Frontal Parietal Squamosal Foramen magnum Dentary Surangular Angular Articular Splenial TAXA MAMMALIA Figures 4-23, 4-24, 4-25 Fig 7-26 (Text) Cat skull (fig. 5.35) Nasal Premaxilla Maxilla Orbit Zygomatic arch Frontal Parietal Occipital bone Jugal (zygomatic) Tympanic bulla Sagittal crest Sagittal suture Coronal suture Condyloid process Occipital condyle Foramen magnum Dentary Canine Incisor Canine Adult Human Skull Same as in whole cat skull with this addition: dentary bones fuse together so well that the whole lower jaw is just called the "mandible" in your illustrations. The term mandible is a "structure" made up of the two dentary bones. The pre-maxillae fuse with the maxillae early in development. The incisors are on the premaxilla and the other upper jaw teeth are on the maxilla. Tympanic bullae are small - called petrous region in your diagrams BIOS 314L – Lab 03 The Postcranial skeleton – vertebrae Very useful vocabulary terms are in table 5.2 AXIAL SKELETON Skeletal elements form along the midline or mid-sagittal axis of the body and are generally endochondral. NOTOCHORD It is a centrally located, gel-filled rod that supports the body of a developing embryo. In most vertebrates it is replaced (partly or completely) by segmentally arranged vertebrae. VERTEBRAE STRUCTURE (figs 5.4, 5.9, 5.11, 5.13, 5.14) The vertebral body or centrum is the large solid disk that takes the compressive forces during body movement. Centra may be flat or have rounded sockets on one side to articulate with adjacent vertebrae. The vertebrae enclose the spinal cord with neural (or vertebral) arches. In the tail (caudal vertebrae) hemal arches enclose blood vessels. Vertebrae form several areas of muscle attachment via neural spines, transverse processes & hemal spines. In fishes, the vertebrae are tightly held together with sheathing of connective tissue or the continuation of the notochord through the vertebrae. Tetrapod vertebrae need more support from the effects of gravity to prevent the back from hanging down in the middle of the trunk. These vertebrae have paired pre (cranial) & post (caudal) zygapophyses that allow vertebrae to articulate with each other, provide additional support, and limit the total range & direction of body movement. The pre-zygapophyses have articulating facets that face upward. They are found on the anterior side of each vertebrae. The post-zygapophyses have articulating facets that face downward. They are found on the posterior side of each vertebrae. Post-zygapophyses fit on top of pre-zygapophyses of interlocking vertebrae. In mammals the zygapophyses begin to curve, so that prezygapophysis facets curl upward & inwardly & the post-zygapophyses curl downward & outwardly. REGIONALIZATION OF VERTEBRAE Fishes have 2 regions in vertebral column: Trunk Vertebrae - with ribs attached. Caudal Vertebrae -form the tail. They have hemal arches & large hemal spines. Anamniote tetrapods have 4 regions in vertebral column: Cervical Vertebra Atlas - its large facets articulate with occipital condyles Its called the atlas because it "supports" the head on its shoulders. Its the only cervical vertebra present in Lissamphibians. Trunk Vertebrae - have small ribs attached in Lissamphibians Sacral Vertebra - only 1 in Lissamphibians. Its larger than trunk vertebrae & attaches to pelvic girdle. Caudal Vertebrae - typically have hemal arches & hemal spines. Amniote tetrapods have 4 or 5 regions in the vertebral column: Cervical Vertebra 1 atlas - its large facets articulate with occipital condyle(s) It tends to enlarge its neural canal, lose its neural spine & possibly lose its centrum 1 axis - it fits into the atlas; often has a large neural spine & it has an odontoid [odon = tooth, oid = like] process (also called the dens) that allows the head to rotate. The cervicals have small ribs in most amniotes. The ribs fuse completely to the cervical vertebrae in birds & mammals. Transverse foramina are visible, but not separate ribs. Trunk Vertebrae - archosaurs, birds & mammals have differentiated trunk vertebrae into the following regions: Thoracic Vertebrae - retain true ribs. They have large neural spines and articulating facets on the sides of the centrum & on the transverse processes where the ribs attach. Lumbar Vertebrae - lack ribs. They have very large transverse processes & no articulating facets. Sacral Vertebra - 2 or more; large & attach to pelvic girdle Caudal Vertebrae - typically have hemal arches & hemal spines. These vertebrae are greatly reduced in size & number in birds & mammals. RIBS They attach to transverse processes on the vertebrae &/or facets on the centrum of the vertebra. STERNUM ONLY in tetrapods, and even then may be missing or very small in a number of tetrapods. For example, the sternum is cartilaginous & tiny in Necturus & won't be seen in our specimens. It acts as an additional brace to attach to support the rib cage or brace the pectoral girdle. MEDIAN FINS Fishes have unpaired, dorsal, anal & caudal fins on the mid-sagittal axis. Some fishes lack anal fins, some have 2 or more dorsal or anal fins. Identification list Fish Vertebrae (fig. 5.4a) Centrum Neural arch Neural spine Basapophyses Dorsal rib Ventral rib Amphibian Vertebrae (fig 5.9, 5.11a) Cervical vertebra (neck) Thoracic vertebrae (trunk) Sacral vertebra Transverse process Neural spine Prezygapophysis Postzygapophysis Turtle Vertebrae (fig 5.12) Cervical vertebrae Rib Capitulum of rib Thoracic vertebrae Sacral vertebrae Caudal vertebrae Bird Vertebrae (fig 5.13) cervical vertebrae prezygapophysis postzygapophysis cervical rib synsacrum caudal vertebrae pygostyle Mammal Vertebrae (fig. 5.14); be able to identify on mounted and disarticulated cats as well as on the odd assortment of loose vertebrae from various species) Atlas Axis Transverse process Transverse foramen Odontoid process (dens) Cervical vertebrae Neural arch Vertebral canal Vertebral body (centrum) Cranial zygapophysis Caudal zygaphphysis Thoracic vertebrae Vertebral arch Spinous process Vertebral canal Vertebral body Cranial zygapophysis Caudal zygaphphysis Rib facet Lumbar vertebrae Pleuropophysis Spinous process Sacral vertebrae Caudal vertebrae Intervertebral disks Intervertebral foramen Vertebral (floating) rib Head tuberculum Costal cartilage (sternal rib) Bios 314L Lab 04 APPENDICULAR SKELETON These are the elements that form the paired, laterally placed appendages. PECTORAL GIRDLE Dermal In fishes a series of bones forms an arc just behind the opercular bones of the skull, e.g. cleithrum, clavicle & several other bones attach the pectoral girdle to the skull. Tetrapods lose many of these bones & lose the attachment to the skull. Thus in tetrapods, the head moves independently of the legs. Tetrapods may retain the clavicles as paired bones that extend from the sternum towards the humerus. Endochondral These elements may be cartilaginous in most fishes & are called scapulocoracoid or coracoscapula. In derived Actinopterygians (Perch), these bones will ossify forming a more ventrally positioned coracoid & a more dorsally positioned scapulae. In tetrapods, these elements may ossify or remain partly cartilaginous: scapula and suprascapula will be dorsal to the humerus & the coracoid will be ventral to the scapula and may attach to the sternum. The glenoid fossa is the socket for articulation with the humerous The endochondral bones hold the pectoral limb in place & tend to dominate or be the largest elements in the pectoral girdle of tetrapods. PELVIC GIRDLE These are endochondral. Fish pelvic girdles are simple rods of cartilage or bone that are suspended in muscle: ischiopubic or pubioischiatic plates. Derived Actinopterygians moved this girdle & its pelvic limbs forward for better braking ability. In some, the pelvic girdle is attached to the pectoral girdle but ventral to the pectoral girdle. ALL tetrapods (with legs) have 3 bones that form the pelvic girdle. pubic bone or pubis - anteriorly oriented (cartilaginous in many anamniotes) ischium - posteriorly oriented ilium - new in tetrapods, oriented dorsally, attaches the pelvic to the sacral vertebra(e) PAIRED LIMBS anterior series - humerus; radius & ulna; carpals; metacarpals; phalanges posterior series - femur; tibia & fibula; tarsals; metatarsals; phalanges MAMMALIAN SCAPULAR STRUCTURES scapula spine- Unique to Therian mammals. It looks like a midline keel down the scapulas superficial surface. The spine increases the surface area for muscles. coracoid process-The only remnant of the posterior coracoid bone. Its size varies in different species. It fused onto the scapula just above the articulation for humerus. acromion process-this is the articulation point for the clavicle. Its a projection at the distal end of the scapular spine HOW TO IDENTIFY INDIVIDUAL MAMMALIAN LONG BONES Be able to identify these individual bones from the front or back legs of diverse mammals. These simple, helpful nontechnical hints may help you know & identify disarticulated mammalian long bones. humerus It has a rounded head at the proximal end, but its head is less uniformly round, and may often be oval shaped or flattened than the head of the femur. The head should be in line with the articulation point at the distal end. radius Its proximal end has a flattened, rounded disc. In ungulates, the radius is greatly enlarged & the rounded disc is less visible. ulna an olecranon process, or "elbow" extends posteriorly behind ulna's articulation with the humerus. The ulna may be large or reduced distally. femur It has a very round head that usually projects off the side of the main shaft on a short "neck". Its distal articulation has 2 large condyles that are ~ at 90 degree angles to the position of the head of the femur. tibia The proximal end is fairly flat, with 2 facets that articulate with the femur's distal paired condyles. The proximal end has a triangular shape because of the anterior ridge that runs down the midline. fibula It articulates with the sides of the tibia so it has 2 small articulation points along the side of the bone, near the top & bottom. The bone is often reduced or fused onto the tibia & its articulations are small & face off to the side of the bone. BIO 314L Lab 05 Comparative Vertebrate Anatomy (Axial & Appendicular) Skeletal System – bones to identify SQUALUS (fig. 5.16, 5.29) Cranial skeleton olfactory/nasal capsule (often broken), orbital capsule (orbit) otic capsule occipital region occipital condyle. basal Appendicular skeleton Pectoral girdle & fin Pelvic girdle & fin TAXA ACTINOPTYERYGII Amia cranial skeleton (fig. 5.30) premaxilla maxilla nasal frontal parietal supratemporal post temporal opercular postinfraorbitals post orbital sub orbital Fish Vertebrae (fig. 5.4a, 5.8) Centrum Neural arch Neural spine Basapophyses Dorsal rib Ventral rib TAXA LISSAMPHIBIA Frog (Rana Cranial skeleton (handout) Premaxilla nasal Frontoparietal Maxilla Pterygoid Orbit Occipital condyle Foramen magnum Vertebrae (fig. 5.9) Cervical vertebra (neck) Thoracic vertebrae (trunk) Sacral vertebra Transverse process Neural spine Pre/post zygapophyses Appendicular skeleton (fig. 5.17) Pectoral girdle & appendage suprascapular cartilage humerus radius/ulna carpals metacarpals phalanges Pelvic girdle & appendage ilium ischium femur tibia/fibula tarsals metatarsals phalanges tarsals metatarsals phalanges TAXON ARCHOSAURIA -- alligator Cranial skeleton (fig. 5.32) Nares Premaxilla Maxilla Prefrontal Frontal Parietal Squamosal Foramen magnum Dentary Surangular Angular Appendicular (fig. 5.18) Pectoral girdle & appendage suprascapular cartilage humerus radius/ulna procoracoid carpals metacarpals phalanges Pelvic girdle & appendage ilium ischium femur tibia/fibula TAXON AVES Vertebrae (fig. 5.13) cervical vertebrae prezygapophysis postzygapophysis cervical rib synsacrum caudal vertebrae pygostyle Appendicular skeleton (fig. 5.20) Sternum Keel Furculum Femur TAXON TESTUDINES Cranial skeleton (fig. 5.33) premaxilla maxilla postorbital supraoccipital prefrontal parietal foramen magnum dentary Turtle Vertebrae (fig. 5.19) Cervical vertebrae Rib Capitulum of rib Thoracic vertebrae Sacral vertebrae Caudal vertebrae Greater trochanter Condyle Tibiotarsus Patella Fibula Tarsometatarsus Hallux Phalanges Scapula Procoracoid Humerous Pneumatic foramen Ulna Olecranon process Radius TAXON MAMMALIA Cranial skeleton (cat; to include other mammals) (fig. 5.35) Nasal Premaxilla Maxilla Orbit Zygomatic arch Frontal Parietal Occipital bone Jugal (zygomatic) Tympanic bulla Sagittal crest Sagittal suture Coronal suture Condyloid process Occipital condyle Foramen magnum Dentary Canine Incisor Canine Phalanx Vertebrae (fig. 5.14) Atlas Axis Transverse process Transverse foramen Odontoid process (dens) Cervical vertebrae Vertebral arch Vertebral canal Vertebral body (centrum) Cranial zygapophysis Caudal zygaphphysis Thoracic vertebrae Vertebral arch Spinous process Vertebral canal Vertebral body Cranial zygapophysis Caudal zygaphphysis Rib facet Lumbar vertebrae Pleuropophysis Spinous process Sacral vertebrae Intervertebral foramina Caudal vertebrae Intervertebral disks Intervertebral foramen Vertebral (floating) rib Head tuberculum Costal cartilage (sternal rib) Pectoral girdle (fig. 5.22, 5.24) scapula coracoid process glenoid fossa scapular spine acromion process metacromion process infraspinous fossa supraspinous fossa clavicle humerus (p. 125) greater tuberculum head lesser tuberculum trochlea capitulum diaphysis epiphysis ulna (p. 127) trochlear notch olecranon styloid process radius manus (p 128) carpals metacarpals phalanges Pelvic girdle pelvis ilium acetabulum ischium pubis femur head greater trochanter lesser trochanter diaphysis epiphysis patella tibia diaphysis epiphysis fibula diaphysis epiphysis calcaneus tarsals metatarsals phalanges BIOS 314L Lab 06 Musculature I Dissect \dis-‘ekt; dỈ-sekt\ [L. dissectus, pp. of dissecare, to cut apart] (1607) 1: to separate into pieces: expose the several parts of (as an animal) for scientific examination. 2: to analyze and interpret minutely While we tend to think of this laboratory exercise as mostly definition #1, we should focus primarily on #2. CUT LESS, OBSERVE MORE. A note on dissection: The primary objective of today and subsequent muscle laboratory exercises is to expose, identify, and study a muscle’s origin, insertion, shape and function. To do so requires patience. I have tried to organize the laboratory such that you will not need to be in a hurry. Some suggestions: The animals you will be working on won’t look like the diagrams. It will take care and effort to clean the muscle and separate it from surrounding ones. Skeletal muscles are typically connected together with fascia, light colored connective tissue. It will take a little effort and patience to carefully remove. Take advantage of other diagrams in addition to your lab manual. You should appreciate the fact that not all animals look alike (inside or out), so spend some time looking at other groups’ dissections as well. The practical exam will be taken off of the animals that you and the other class members dissect. Thus, it is to your best interest to take your time and do a good job. You should appreciate the fact that good dissection is a motor skill that must be acquired. It will take time to develop the skills necessary to be accomplished at separating the individual muscles and muscle fibers. Use the scalpel very sparingly. Scissors are only necessary to cut skin and to transect across the belly of the muscle. Most muscles can be separated with a blunt probe and a bit of patience. To identify a muscle: 1. Read the description in your lab manual of the muscle’s location and function. 2. Locate the reference points on your animal 3. Look for the muscle boundaries 4. Verify the muscle boundaries with gentle, judicious use of the blunt probe 5. Finally, use the blunt probe and forceps to remove fascia and fat around the muscle. In today’s lab, we will be looking at the superficial axial, pectoral and hypobranchial and branchiomeric musculature of Necturus and Felis (the mudpuppy and the cat) You will find that amphibians, by comparison are notoriously easy to remove the skin. There is much less connective tissue between the skin and the fascia. However, the muscles are smaller and identification more difficult. With Necturus, you will carefully dissect and discard the skin off the entire animal before proceeding. Do so slowly; care should be taken not to pull muscles off while removing the skin. While using scissors, cut away from the animal taking care not to damage the underlying musculature. While there is certainly more than one way to skin a cat, most of them are bad ways. Our goal in the mammal dissections is to keep the skin attached to some degree (in order to keep the muscles damp). I would suggest: 1. Make a careful incision along the ventral midline from groin to chin. When cutting through the skin, make sure your scissors are pointed away from the animal so not to damage the internal anatomy. 2. On the left side, extend the mid-ventral incision laterally along the fore- and hind limbs to the base of the paws. 3. Cut circularly around the base of the paws 4. Now the skin can carefully be freed from the underlying tissue all the way to the dorsal midline. 5. For portions of today’s lab, you will additionally want to make an incision along the ventral throat midline and reflect the skin up along the face, just caudal to the eye and along the dorsal midline 6. Try to not let your specimen dry out. There are spray bottles of wetting solution around. Use them! In today’s lab, we will focus mostly on the axial, pectoral and head and neck musculature. As in the past weeks, we may toss out some of these terms that I’m asking you to identify. However, what follows is a list that I hope we will be able to work through, as they represent much of the fundamental musculature: Necturus (fig. 6.11, 6.12, 6.14) Ventral pectoral girdle, forelimb, and throat pectoralis rectus abdominus (just caudal – originates on the linea alba). Together, these two muscles converge laterally on the humerus. supracoracoideus procoracohumeralis – inserts proximally on the humerus rectus cervicis – the ventral neck muscles humeroantebrachialis (anterior to) coracobrachialis – upper arm muscles intermandibularis – thin sheet overlying the anterior ventral throat interhyoideus – often indistiguishable from intermandibularis; posterior throat on one side, cut through the intermandibularis/interhyoideous. below should be able to identify the geniohyoideus branchiohyoideus lateral pectoral girdle, forelimb and lateral head dorsalis scapulae and latissimus dorsi – converges onto shoulder near scapula and humerus dorsalis trunci triceps brachii forearm extensors and flexors depressor mandibulae levator mandibulae externus levator mandibulae anterior Felis (fig. 6.15, 6.16, 6.17) be able to identify the function and/or the origin and insertion, as well as the location of: superficial pectoral musculature pectoralis pectoralis superficialis (pectoralis major of humans) pectoralis profundus (pectoralis minor of humans) cleidobrachialis (palpate the clavicle within) (lateral view works better here) trapezious thoracic trapezius (thin sheet covering latissimus dorsi) cervical trapezius latissimus dorsi sternomastoid triceps brachii forearm extensors forearm flexors head and neck musculature sternomastoid (be careful with the jugular vein not to cut it) sternohyoid masseter temporalis digastric BIOS 314L Lab 07 Entire Vocabulary list for external musculature Necturus (fig. 6.11, 6.12, 6.14) Ventral pectoral girdle, forelimb, and throat pectoralis rectus abdominus (just caudal – originates on the linea alba). Together, these two muscles converge laterally on the humerus. supracoracoideus procoracohumeralis – inserts proximally on the humerus rectus cervicis – the ventral neck muscles humeroantebrachialis (anterior to) coracobrachialis – upper arm muscles intermandibularis – thin sheet overlying the anterior ventral throat interhyoideus – often indistiguishable from intermandibularis; posterior throat on one side, cut through the intermandibularis/interhyoideous. below should be able to identify the geniohyoideus branchiohyoideus lateral pectoral girdle, forelimb and lateral head dorsalis scapulae and latissimus dorsi – converges onto shoulder near scapula and humerus dorsalis trunci triceps brachii forearm extensors and flexors depressor mandibulae levator mandibulae externus levator mandibulae anterior axial musculature, pelvic girdle (fig 7-17, 7-18) epaxial musculature hypaxial musculature rectus abdominis puboischiofemoralis externus puboischiotibialis leg extensors leg flexors dorsalis trunci Felis be able to identify the function and/or the origin and insertion, as well as the location of: superficial pectoral musculature (fig. 6.15, 6.17) pectoralis pectoralis superficialis (pectoralis major of humans) pectoralis profundus (pectoralis minor of humans) cleidobrachialis (palpate the clavicle within) (lateral view works better here) trapezious latissimus dorsi sternomastoid triceps brachii forearm extensors forearm flexors head and neck musculature (fig. 6.16, 6.17, 6.19) sternomastoid (be careful with the jugular vein not to cut it) sternohyoid masseter temporalis digastric trunk musculature (fig. 6.15, 6.17) external oblique latissimus dorsi pelvic and thigh musculature (figs. 6.22, 6.23) gluteus medius/superficialis biceps femoris semitendinosus gracilis sartorius gastrocnemius extensor digitorum longus tendons of extensor digitorum longus tibialis anterior BIOS 314L Circulation Lab 08 Objectives 1. Be able to trace the movement of blood in Squalus from the heart to the respiratory surfaces (gills) and back to the heart. Be able to describe the basic flow of blood in Squalus 2. Recognize and identify the heart and its major vessels in Felis. Be able to describe the basic flow of blood through a ‘typical’ mammal 3. Trace the flow of blood through the mammalian heart and dissect and identify the major components of the heart. 1. Gill circulation in Squalus Follow the dissection guide starting on p. 140 of your lab manual. You will want your dissection to roughly correspond with the figure on p. 142. Once the pharynx has been carefully opened, turn to p. 141. Read through this as you dissect and identify the terms listed below. Useful diagrams (including handouts): Fig 8.2, 8.4 – ventral view of heart and afferent arteries Fig 8.3 – branchial arches Identification Sinus venosus Atrium Ventricle Conus arteriosus Ventral aorta Afferent branchial arteries (1-5) Efferent branchial arteries Dorsal aorta 2. The heart and its major vessels in Felis Follow the dissection guide starting on p. 151. Retract and/or remove the rib cage and expose the cat similar to fig. 8.10 & 8.11 on p. 153/1154. Identification Rt. Atrium Rt. Ventricle Coronary vein Coronary artery Pulmonary trunk Left ventricle Left atrium Pulmonary arteries/veins Aortic arch Descending aorta Brachiocephalic artery Common carotid artery External jugular vein Subclavian vein Brachiocephalic vein Cranial (pre) vena cava Caudal (post) vena cava 3. Internal structure of the heart. See Fig 8.7, p. 148 Identification Pulmonary semi-lunar valve Tricuspid valve (rt. A-V) Bicuspid (left A-V) Chordae tendineae Interventricular septum Semilunar valves Be able to trace the flow of blood through the heart. Bios 314/514 Lab 09 peripheral vasculature Last week, we identified the pump (heart) and the major entrances and exits. This week, we want to further identify the peripheral vasculature. Since we’re done with the muscles, you may find it helpful to start carefully removing the musculature. Find the cranial vena cava. Coming into this, locate: subclavian vein axillary vein subscapular vein internal jugular vein external jugular vein maxillary (posterior facial) linguofacial (anterior facial) transverse jugular (may be broken) Find the caudal vena cava. As it passes through the diaphragm, identify the phrenic vein. renal vein ovarian vein (often not found in these preps) external and internal iliac vein femoral lumbar Find the aortic arch. From that, there are two branches, the brachiocephalic and left subclavian. Figs. 8.10, 8.11, 8.13 From the brachiocephalic follow and identify: left carotid artery right carotid artery right subclavian artery brachial artery axillary artery subscapular Find the dorsal aorta. From there, identify celiac artery renal artery ovarian artery lumbar external iliac artery internal iliac artery femoral Lab 10 Urogenital system From identifying the peripheral circulation last week, you should be relatively familiar with the vasculature surrounding the urogenital system. Review last week’s lab, and follow the circulation into the renal and reproductive systems. Squalus (Fig 9.3, 9.4) The pelvic fins of males posses enlarged medial bars, the claspers, derived from pterygiophores. The medial side bears a groove that carries seminal fluid during copulation. Nearly all of our Squalus specimens are males. In your shark, remove and discard the liver by cutting the lobes. Then identify the location and function of the following: Testis Ductus deferens (deposits into archinephric duct) Seminal vesicles Sperm sac Leydig’s gland Kidney (cut through parietal peritoneum) Mesonephric duct (drains kidney) Cloaca Necturus (Figs. 9.5, 9.6) The kidneys and the arrangement of the associated ducts are similar in Squalus and Necturus. Both the shark and the mudpuppy posess opisthonephric kidneys. As in the male shark, the ductus deferens serves in transport of sperm and seminal fluids. Unlike the shark, Necturus has urinary collecting tubules to transport urine from the kidneys into the ductus deferens. In addition to the components described above, identify in your mudpuppy the testis, epididymus, urinary bladder, and cloaca. Felis (Fig 9.7, 9.8) – everyone probably has female Felis….some folks hopefully still have the urogenital system intact. We may need to share cats today, as some folks have already removed the reproductive tracts. From the hilus (medial indentation of kidney), trace ureter down to urinary bladder. Arising from the medial indentation (hilus) on each kidney is a thin white tube, the ureter that passes posteriorly to the base of the muscular urinary bladder. The bladder narrows into the urethra. Be careful to not damage the uterus when tracing the location of the ureters. There is generally a great deal of fat lying within the ovaries and reproductive tract. To follow the reproductive tract further, cut through the pubic and ishial symphyses with heavy scissors. Spread the cut by pressing the knees of the cat laterally. From the urethra and vaginal vestibule, trace the reproductive tract back to the body of the uterus. Identify the uterine horns, and, if injected properly, the oviducts and ovaries. the mammalian kidney In lecture, we will talk much more about the internal anatomy and design of the vertebrate kidney. For this part of the lab, I want you to become familiar with the internal and external anatomy (Fig 9.8; handout). in the larger kidneys provided, recognize and identify the ureter and hilus of the kidney. Carefully section the kidney into two halves and identify the cortex, medulla, renal pelvis. You should be able to identify the renal artery and its vasculature. We will talk in lecture about the flow of blood through the kidney and functional anatomy. Introduction to the Nervous System Bios 314L Lab 11 Today's Objectives: 1) identify the major divisions of the brain and compare across Squalus and Homo 2) compare the relatives sizes and proportions of the human and shark brain The human brains are on the cart and available to you. PLEASE BE CAREFUL IN HANDLING THEM!!! To dissect the Squalus (fig. 10.6, 10.7), most of the roof of the chondrocranium will have to be removed. Do so carefully and don't cut the white 'strings' that pass through the various foramen. a) remove the skin over the head and snout b) clear away the watery tissue and remove the upper eyelids c) be careful not to damage the superficial opthalmic nerve if possible d) pick away the chondocranium near the orbit e) remove the skin around the spiracle, be careful not to damage the hyomandibular nerve along the posterior of the spiracle f) remove enough of the axial muscles to the chondrocranium to expose it g) carefully pick away the roof of the chondocranium h) carefully remove the sides. be careful not to damage the soft brain tissue or the nerves! i) dissect out the first few spinal nerve roots j) carefully free and remove the entire brain and first part of the spinal cord if you're not sure what to do let me help you with this part! Note: there are human brains already in mid sagittal section. DO NOT cut any human brains!! However, you may cut the sheep brain directly down the longitudinal fissure (fig. 10.12, 10.13, 10.14) Identify: SHARK & Necturus BRAIN olfactory lobe telencephalon (cerebrum) thalamus hypothalamus pons optic lobe optic chiasms cerebellum medulla Cranial nerves I, II, V, VI, VII-IX spinal cord dorsal root ventral root SHEEP & HUMAN BRAIN (*only) olfactory lobe telencephalon (cerebral hemispheres) frontal, parietal, temporal, & occipital* lobes of the cerebrum central sulcus (btwn frontal & parietal) thalamus hypothalamus pons optic lobe cerebellum medulla inferior colliculus superior colliculus (optic tectum) corpus callosum optic chiasm Questions to answer: 1) what is the relative proportion of brain sizes for the following: Squalus optic tectum vs. Homo superior colliculus Squalus cerebellum vs. Homo vs. Sheep cerebellum? Squalus cerebrum vs. Homo cerebrum 2) why the differences in proportion? 3) what is the difference in function between the dorsal and ventral roots of the spinal cord 4) where might information travel to get from one hemisphere of the mammal brain to the other? 5) what is the difference in shape between the human cerebellum and the shark? why? Nervous System II Bios 314L Objectives: 1. Identify and recognize the subdivisions and further anatomical components of the vertebrate brain 2. Identify the three dimensional arrangement of the internal components of the telencephalon and diencephalon of the sheep and human 3. Identify the size differences and reason for them, in the mammalian spinal cord 4. Identify the internal components of the sheep kidney (that we didn’t get done a couple of weeks ago). 5. Review for lab practical. 1. Attached to this handout are two pages of worksheets. Identify the unlabeled components and make sure you can identify the corresponding components on the shark, cat, sheep and human. 2. Get another sheep brain from the brain bucket. One member of your group will make a series of horizontal sections, roughly 1/4” thick with the brain knife (don’t use your scalpel, it won’t cut smooth enough to recognize the info you’ll need). Identify: Gray matter White matter Lateral ventricle Third ventricle Internal capsule Thalamus Caudate Putamen Compare these structures with the human sections (either the preserved ones or the ones encased in plastic). 3. Assuming my dissections of the cat brain and spinal cord were successful, otherwise use the human spinal cord, identify where the spinal cord begins and the medulla ends Cervical spinal cord Cervical enlargement Thoracic spinal cord Lumbar enlargement Sacral spinal cord Why are there differences in size and shape for these different regions?