Chemistry Minilab NaClO3 and C12H22O11
advertisement
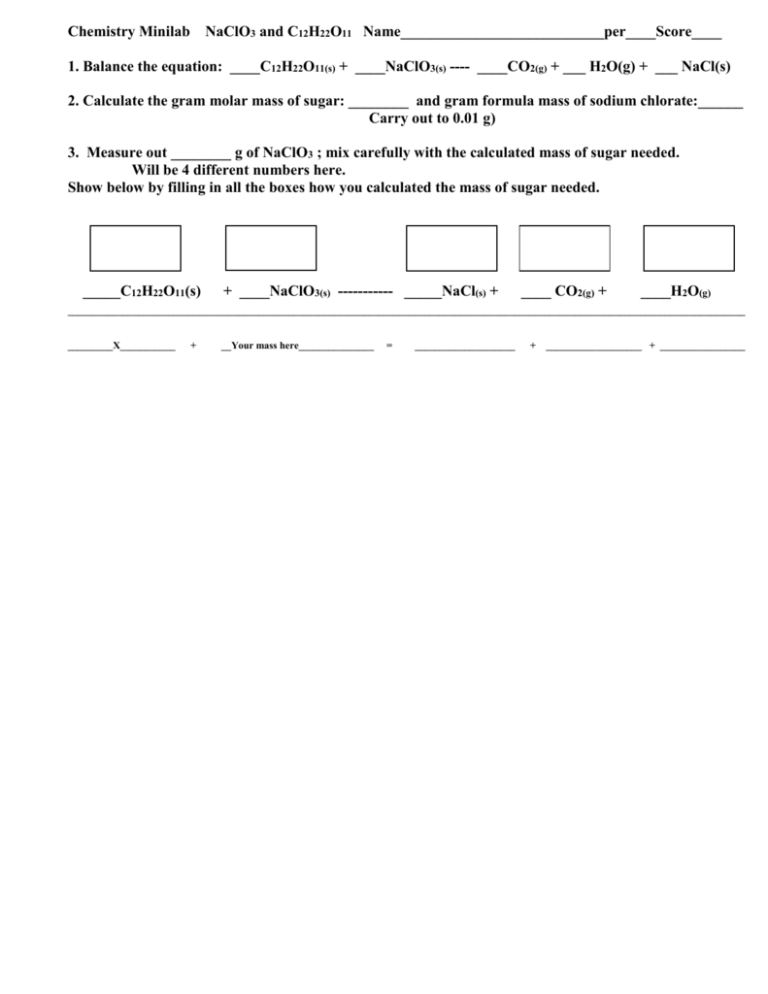
Chemistry Minilab NaClO3 and C12H22O11 Name___________________________per____Score____ 1. Balance the equation: ____C12H22O11(s) + ____NaClO3(s) ---- ____CO2(g) + ___ H2O(g) + ___ NaCl(s) 2. Calculate the gram molar mass of sugar: ________ and gram formula mass of sodium chlorate:______ Carry out to 0.01 g) 3. Measure out ________ g of NaClO3 ; mix carefully with the calculated mass of sugar needed. Will be 4 different numbers here. Show below by filling in all the boxes how you calculated the mass of sugar needed. _____C12H22O11(s) + ____NaClO3(s) ----------- _____NaCl(s) + ____ CO2(g) + ____H2O(g) _______________________________________________________________________________________________________________________________________ _________X___________ + __Your mass here_______________ = ____________________ + ___________________ + _________________










