Chapter 11. Liquids, Solids
advertisement

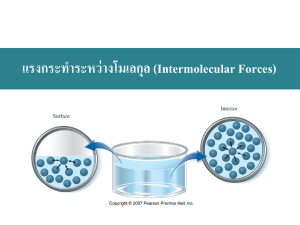
Chapter 11: Liquids, Solids, & Intermolecular Forces I) Introduction & Review 1. There are three phases of matter: gases, solids, and liquids. Solids & Liquids are known collectively as condensed phases; due to the close proximity of the constituent particles that make up the solid or liquid. 2. General Characteristics of Gases, Liquids, & Solids (Table 11.1, 11.2) A) Gases - (Review Chapter 5) Characterized by variable shape and volume. Component particles far apart and are in rapid motion; consequently, molecules interact very little with each other. Low density Highly compressible. B) Solids - Particles are in a fixed rigid structure (fixed shape & volume). Since particles are very close together they exhibit strong interactions with one another. Solids have highest densities and are not compressible. Solids may be crystalline or amorphous depending on whether the atoms are arranged in a well-ordered three dimensional array (crystalline) or have no long-range order (amorphous). C) Liquids - Liquids have a definite volume; but an indefinite shape Intermediate densities (greater than gases; less than solids) Not easily compressed 3. Bonding & Polarity: A Review A) Polar covalent bonds form between atoms of differing electronegativities. This results in the formation of a bond dipole. B) A bond dipole is characterized as having one end with negative character (charge) and one end with positive character (charge). C) A dipole is represented by an arrow with a cross at one end ( | ) where the point of the arrow represents the negative end of the dipole ( -) and the crossed end represents the positive end ( +). 2 D) A molecule that is polar must contain a net dipole moment. Recall that a truly symmetrical molecule is nonpolar by definition; therefore, it does not have a dipole moment. E) Dipole moment ( ) is calculated using the relationship = Q * r II) Intramolecular versus Intermolecular Forces 1. Definitions A) Intramolecular Forces of attraction occur within molecules (occur between valence shells of the atoms that make up a molecule). B) Intermolecular Forces of attraction occur between molecules and atoms; these forces account for the different phases found in matter, and for the manner in which certain molecules behave (proteins, DNA, etc.). 2. Changes in state for a substance are due to changes in the forces among the molecules rather than those within the molecules. Molecules remain intact as one goes from a solid to a liquid to a gas. 3. Types of Intermolecular Forces (also called Van der Waals forces) A) Ion-dipole forces result from the electrical interactions between an ion and the partial charges on a polar molecule. - Anions are oriented toward the positive end of a dipole; while cations are near the negative end of a dipole. - Ion-dipole forces are often associated with ionic salts. B) Dipole-dipole interactions occur with polar molecules. Polar molecules have a dipole moment; hence they can attract each other electrostatically. - Direct Correlation of with boiling points (bp) C) London dispersion forces are found in all molecules but are most prominent in nonpolar molecules and noble gas atoms. - London forces occur when instantaneous dipoles are formed within a nonpolar framework. This phenomenon is termed polarizability and is found to occur most often with large nonpolar atoms or large bulky groups of atoms. D) Hydrogen bonding is a special type of dipole-dipole interaction in which hydrogen is bonded to a small highly electronegative atom(like N, O, and F). 3 - This results in very strong intermolecular forces and accounts for the unusually high boiling points for H2O and NH3. E) Review Table 11.4 III) The Liquid State 1. General Properties of Liquids (A Review) A) low compressibility B) lack of rigidity (liquids can assume shape of container) C) higher densities when compared to gases 2. Properties associated with Intermolecular Forces A) Surface tension – resistance of a liquid to spread out and increase its surface area. B) Viscosity is a measure of the resistance to flow for a liquid. Liquids that are highly viscous tend to either have large attractive forces, or have complex structures. IV) Phase Changes 1. Kinetic Molecular Theory (Re: Chapter 5) tells us that molecules acquire more kinetic energy (move faster) as the temperature increases. 2. If molecules possess sufficient energy, they can transition from one phase of matter into another (Example: ice melting to form liquid water). This transition from one phase of matter to another is termed a phase change. 3. Every phase change is governed by the equation free energy equation: G = H - TS where H is the change in enthalpy, S is the change in entropy and T is the kelvin temperature. 4. Phase changes may be endothermic or exothermic depending upon whether the system absorbs or releases heat energy. Recall the sign convention for H for exothermic versus endothermic systems. 5. Additionally we are also concerned with S for a given phase change. Entropy is the thermodynamic property that deals with the randomness or disorder of a system. - Gases have the highest entropy, while solids have the lowest (gases are the most disordered phase of matter; solids are the most ordered). 6.Changes of state are physical changes, one is changing the form that a sample of matter exists in, not its composition. 4 7.If sufficient energy is supplied to a sample, causing the substance to be broken down into its component atoms, then that is a chemical change. V) Vapor Pressure & Changes of State 1. Vaporization is the process by which a liquid is converted into a gas. It is endothermic since energy is needed to overcome intermolecular forces found in the liquid. A) Heat of vaporization ( Hvap) is energy required to vaporize 1 mole of liquid at a pressure of 1 atm. 2. Condensation is the reverse process (involves converting a gas into a liquid). Not surprisingly, it is exothermic. 3. In a closed system, the rate of evaporation & condensation will reach a point where they are equal. Such a system is said to be in equilibrium. Although there appears to be no net observable change, the equilibrium system is highly dynamic on the molecular level. 4. The pressure of the vapor that is present @ equilibrium is called the equilibrium vapor pressure or more commonly the vapor pressure of the liquid. A) Volatile liquids have high vapor pressures. Liquids with low vapor pressures are said to be nonvolatile. B) As the degree of intermolecular forces increases, the vapor pressure of the corresponding liquids decrease. A) Vapor pressure increases significantly with increasing temperature. B) To calculate vapor pressures one would use the following equations: (Clausius-Clapeyron Equation) P atmosphere = P vapor + P Hg column ln(P vap) = - ( H vap / R) (1 / T) + C ln(P vap, T1 / P vap, T2) = ( H vap / R) (1 / T2 - 1 / T1) 5. Sublimation is the process by which a solid can be converted directly into a gas. Such a substance would have a characteristic vapor pressure. Sublimation is an endothermic process. 6. The temperature at which boiling occurs when the external pressure is exactly 1 atm is called the normal boiling point of the liquid. 5 VI) The Solid State 1. Introduction to Structures & Types of Solids A) Amorphous solids have considerable disorder in their atomic arrangement. - Glass & Graphite are examples of amorphous solids. B) Crystalline solids have a highly regular atomic arrangement - Types of Crystalline Solids o Ionic Solids All Salts are Ionic Compounds Ions found at points of the crystal lattice Stable, high melting substances that are held together electrostatically. o Molecular Solids Examples are sugar and ice Discrete covalently bonded molecules found at points of crystal lattice Characterized by strong covalent bonding within the molecules but weak forces between molecules. o Network Solids Very large molecules whose atoms are linked together by covalent bonds into a threedimensional network. Elements C and Si form network solids. i. Carbon 1. Diamond 2. Graphite 3. Fullerenes ii. Silicon 1. Silica 2. Glass 3. Ceramics 6 o Metallic Solids Atoms found at lattice points Properties found in metals can be traced back to the nondirectional covalent bonding found in metallic crystals. High thermal & electrical conductivity Malleable – metals can be pounded into thin sheets Ductile – metals can be pulled into wires 2. X-ray Diffraction: Probing the Structure of Solids A) Structure of crystalline solids determined using X-ray diffraction. B) Bragg equation n = 2d sin - Used to test predictions of molecular structure & geometry & to determine the structure of very complex molecules (proteins, enzymes, etc.). 3. Unit Cells and Packing of Spheres in Solids A) Atoms within a crystal are found in a lattice (3D system of points that show the positions of the atoms, molecules, or ions that make up the substance). B) The smallest repeating unit of the lattice is called the unit cell. A crystal is generated by repeating the unit cell in all three dimensions. - Types of Unit Cells o Simple Cube o Body Centered Cube o Face Centered Cube C) Closest Packing Model for Metallic Crystals - Model assumes that metal atoms that make up a crystal are uniform, hard spheres. - Atoms are packed in as uniform a fashion as possible. - Two arrangements are possible o Hexagonal closest packing (hcp) Atoms arranged in ababab layers o Cubic closest packing (ccp) Atoms arranged in abcabcabc layers 7 4. Phase Diagrams A) Pictorial representation of phases of matter; it is a plot of pressure as a function of temperature. B) See Figures for phase diagrams corresponding to water & carbon dioxide. C) Be able to distinguish between phases of matter in a phase diagram. D) Be familiar with terms like normal boiling point, normal melting point, critical point, & triple point.