02-06 Average Atomic Mass Worksheet 2
Average Atomic Mass Worksheet 2 Name: ______________________________
Show your work for each of the following problems.
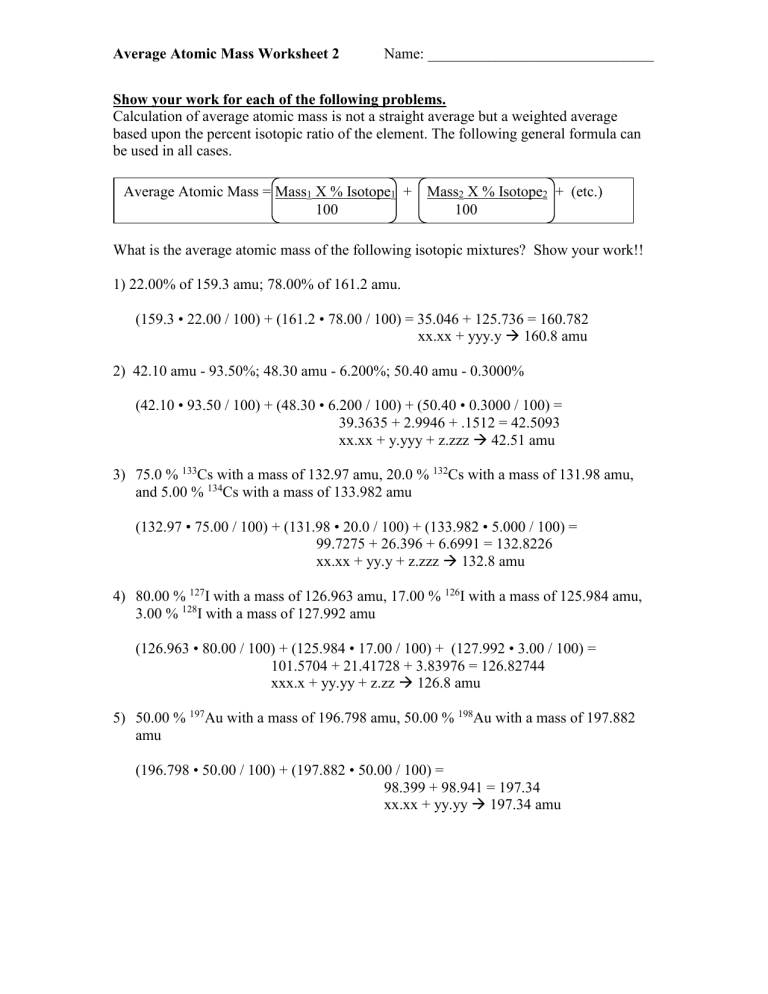
Calculation of average atomic mass is not a straight average but a weighted average based upon the percent isotopic ratio of the element. The following general formula can be used in all cases.
Average Atomic Mass = Mass
1
X % Isotope
1
+ Mass
2
X % Isotope
2
+ (etc.)
100 100
What is the average atomic mass of the following isotopic mixtures? Show your work!!
1) 22.00% of 159.3 amu; 78.00% of 161.2 amu.
(159.3 • 22.00 / 100) + (161.2 • 78.00 / 100) = 35.046 + 125.736 = 160.782
xx.xx + yyy.y
160.8 amu
2) 42.10 amu - 93.50%; 48.30 amu - 6.200%; 50.40 amu - 0.3000%
(42.10 • 93.50 / 100) + (48.30 • 6.200 / 100) + (50.40 • 0.3000 / 100) =
39.3635 + 2.9946 + .1512 = 42.5093 xx.xx + y.yyy + z.zzz
42.51 amu
3) 75.0 % 133 Cs with a mass of 132.97 amu, 20.0 % 132 Cs with a mass of 131.98 amu, and 5.00 %
134
Cs with a mass of 133.982 amu
(132.97 • 75.00 / 100) + (131.98 • 20.0 / 100) + (133.982 • 5.000 / 100) =
99.7275 + 26.396 + 6.6991 = 132.8226 xx.xx + yy.y + z.zzz
132.8 amu
4) 80.00 %
127
I with a mass of 126.963 amu, 17.00 %
126
I with a mass of 125.984 amu,
3.00 % 128 I with a mass of 127.992 amu
(126.963 • 80.00 / 100) + (125.984 • 17.00 / 100) + (127.992 • 3.00 / 100) =
101.5704 + 21.41728 + 3.83976 = 126.82744 xxx.x + yy.yy + z.zz
126.8 amu
5) 50.00 % 197 Au with a mass of 196.798 amu, 50.00 % 198 Au with a mass of 197.882 amu
(196.798 • 50.00 / 100) + (197.882 • 50.00 / 100) =
98.399 + 98.941 = 197.34 xx.xx + yy.yy
197.34 amu
Average Atomic Mass Worksheet 2 Name: ______________________________
6) 15.35 %
55
Fe with a mass of 54.997 amu, 84.65 %
56
Fe with a mass of 55.989 amu
(54.997 • 15.35 / 100) + (55.989 • 84.65 / 100) =
8.4420395 + 47.3946885 = 55.836728 x.xxx + yy.yy
55.84 amu
7) 99.00 % 1 H with a mass of 1.0003 amu, 0.8000 % 2 H with a mass of 2.0074 amu,
0.2000 %
3
H with a mass of 3.0121 amu
(1.0003 • 99.00 / 100) + (2.0074 • 0.8000 / 100) + (3.0121 • 0.2000 / 100) =
0.990297 + 0.0160592 + 0.0060242 = 1.0123804
0.xxxx + 0.0yyyy + 0.00zzzz
1.0124 amu
8) Find the average atomic mass for Li if 7.50% of Li atoms are 6 Li with a mass of
6.0151223 amu and 92.5% are
7
Li with a mass of 7.0160041 amu.
(6.0151223 • 7.50 / 100) + (7.0160041 • 92.5 / 100) =
0.4511341725 + 6.489803793 = 6.940937965
0.xxx + y.yy
6.94 amu
9) Find the average atomic mass for B if 19.9% of B atoms are
10
B with a mass of
10.0129371 amu and 80.1% are
11
B with a mass of 11.0093055 amu.
(10.0129371 • 19.9 / 100) + (11.0093055 • 80.1 / 100) =
1.992574483 + 8.818453706 = 10.81102814 x.xx + y.yy
10.81 amu
10) Find the average atomic mass for Cl if 75.78% of Cl atoms are
35
Cl with a mass of
34.96885271 amu and 24.22% are
37
Cl with a mass of 36.96590260 amu.
(34.96885271 • 75.78 / 100) + (36.96590260 • 24.22 / 100) =
26.49939658 + 8.95314161 = 35.45253819 xx.xx + y.yyy
35.45 amu
11 Find the average atomic mass for Mg if 78.99% of Mg atoms are
24
Mg with a mass of
23.9850419 amu, 10.00% are
25
Mg with a mass of 24.9858370 amu, and 11.01% are
26 Mg with a mass of 25.9825930 amu.
(23.9850419 • 78.99/100) + (24.9858370 • 10.00/100) + (25.9825930 • 11.01 / 100) =
18.9457846 + 2.4985837 + 2.860682489 = 23.40505079 xx.xx + y.yyy + z.zzz
23.41 amu
Average Atomic Mass Worksheet 2 Name: ______________________________
12) There are 2 isotopes of copper that occur naturally;
63
Cu and
65
Cu. The
63
Cu atoms which occur 77.93 % of the time have a mass of 62.929601 amu and the
63
Cu atoms have a mass of 64.927794 amu.
(62.929601 • 77.93% / 100) + (64.927794 • (100–77.93%) / 100) =
49.04103806 + 14.30749414 = 63.3485322 xx.xx + yy.yy
63.35 amu
13) There are 2 isotopes of gallium that occur naturally;
69
Ga and
71
Ga. The
69
Ga atoms which occur 19.32% of the time have a mass of 68.925581 amu and the
71
Ga atoms have a mass of 70.924707 amu.
(68.925581 • 19.32 / 100) + (70.924707 • (100–19.32) / 100) =
13.31642225 + 57.22205361 = 70.53847586 xx.xx + yy.yy
70.54 amu
14) Rubidium has two common isotopes,
85
Rb and
87
Rb. If the abundance of
85
Rb which has an atomic mass of 84.974 amu is 72.2% and the abundance of
87
Rb which has an atomic mass of 86.973 amu is 27.8%, what is the average atomic mass of rubidium?
(84.974 • 72.2 / 100) + (86.973 • 27.8 / 100) =
61.351228 + 24.178494 = 85.529722 xx.x + yy.y
85.5 amu
15) Uranium has three common isotopes. If the abundance of
234
U which has an atomic mass of 233.878 amu is 0.01%, the abundance of
235
U which has an atomic mass of
234.892 amu is 0.71%, and the abundance of
238
U which has an atomic mass of
237.911 amu is 99.28%, what is the average atomic mass of uranium?
(233.878 • 0.01 / 100) + (234.892 • 0.71 / 100) + (237.911 • 99.28 / 100) =
0.233878 + 1.6677332 + 236.1980408 = 237.8891618
0.x + y.y + zzz.z
237.9 amu
16) Titanium has five common isotopes:
46
Ti (8.00%, 45.983 amu),
47
Ti (7.80%, 46.988 amu),
48
Ti (73.4%, 47.910 amu),
49
Ti (5.50%, 48.923 amu),
50
Ti (5.30%, 49.942 amu). What is the average atomic mass of titanium?
(45.983 • 8.00 / 100) + (46.988 • 7.80 / 100) + (47.910 • 73.4 / 100) + (48.923 • 5.50 /
100) + (49.942 • 5.30 / 100) =
3.67864 + 3.665064 + 35.16594 + 2.690765 + 2.646926 = 47.847355 v.vv + w.ww + xx.x + y.yy + z.zz
47.8 amu
Average Atomic Mass Worksheet 2 Name: ______________________________
17) What is the average atomic mass of silver if 51.84% of silver atoms have a mass of
106.905 amu and 48.16% of them have a mass of 108.905 amu?
(106.905 • 51.84 / 100) + (108.905 • 48.16 / 100) =
55.419552 + 52.448648 = 107.8682 xx.xx + yy.yy 107.87 amu
18) Find the average atomic mass of krypton using the data below:
Isotope Mass (AMU)
77.920
Percentage Abundance (%)
0.350
79.916
81.913
82.914
83.912
2.27
11.56
11.55
56.90
85.911 17.37
(77.920 • 0.350 / 100) + (79.916 • 2.27 / 100) + (81.913 •11.56 / 100) + (82.914 •
11.55 / 100) + (83.912 • 56.90 / 100) + (85.911 • 17.37 / 100) =
0.27272 + 1.8140932 + 9.4691428 + 9.576567 + 47.745928 + 14.9227407 =
83.8011917
0.uuu + v.vv + w.www + x.xxx + yy.yy + zz.zz
83.80 amu
19) Calculate the average atomic mass of chromium, given the following percent abundances and isotopic masses.
Isotope Mass (AMU)
49.946
51.941
Percentage Abundance (%)
4.345%
83.789%
52.941
53.939
9.501%
2.365%
(49.946 • 4.345 / 100) + (51.941 • 83.789 / 100) + (52.941 • 9.501 / 100) + (53.939 •
2.365 / 100) =
2.1701537 + 43.52084449 + 5.02992441 + 1.27565735 = 51.99657995 w.www + xx.xxx + y.yyy + z.zzz 51.997 amu
20) Calculate the average atomic mass of indium if 4.3% of its atoms are indium-113 with an atomic mass of 112.941 amu and 95.7% of its atoms are indium-115 with an atomic mass of 114.943 amu.
(112.941 • 4.3 / 100) + (114.943 • 95.7 / 100) =
4.856463 + 110.000451 = 114.856914 x.x + yyy.
115 amu
Average Atomic Mass Worksheet 2 Name: ______________________________
21) Explain why atoms have different isotopes. In other words, how is it that helium can exist in three different forms?
Neutrons have no charge, so they may exist in a nucleus without affecting the charge on the atom. Neutrons serve as buffers between the protons in the nucleus. Otherwise, there would be many positive charges all bundled together. All these positive charges would have the effect of repelling each other. That is why the number of neutrons grows as the number of protons increases. Since there are different physical configurations that protons and neutrons could get into, there are different isotopes.
An example with helium would be a two dimensional representation as shown below of 2 protons and 2 neutrons (atomic mass of 4):
+
0 0
+
Another possibility would be a three dimensional representation with a triangle made of 3 neutrons, with one proton above and one below the triangle. This would have an atomic mass of 5.







