EMERGENCY DRUGS
advertisement

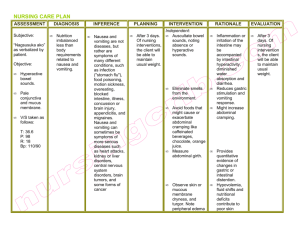
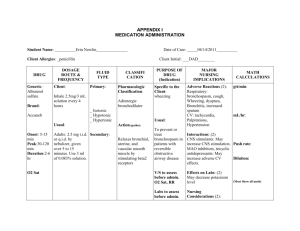
EMERGENCY DRUGS Advise to use hard candy, ice chips, etc. for dry mouth. CARDIAC DRUGS ATROPINE SULFATE Isopto Atropine Classification Anticholinergics Dosage Bradycardia: 0.5 mg IV every 3-5 mins, max of 0.04 mg/kg Cardiac Arrest: 1 mg every 3-5 mins Nerve and Organophosphate symptoms: may repeat in 2 mg increments q 3 mins titrated to relief symptoms Indication Pre-op meds/pre-anesthetic meds To restore cardiac rate and arterial pressure during anesthesia when vagal To lessen the degree of A-V heart block To overcome severe carotid sinus reflex Antidote for cholinergic toxicity Side effects CNS: restlessness, ataxia, disorientation, hallucinations, delirium, coma, insomnia, agitation, confusion. CV: tachycardia, angina, arrhythmias, flushing. EENT: photophobia, blurred vision, mydriasis. GI: dry mouth, constipation, vomiting. GU: urine retention. Hematologic: leukocytosis Other: anaphylaxis Adverse effects CNS: headache, excitement. CV: palpitations GI: thirst, nausea NITROGLYCERINE Nitrostat Classification Antianginal Nitrate Vasodilator, Coronary Dosage 0.3-0.4 mg SL q 5 min, max 3 doses Every 6 hrs except for midnight (cream) Wear 12 hrs a day for skin patch Action Relaxes the vascular smooth system Reduces myocardial oxygen consumption Reduces left ventricular workload Reduces arterial BP Reduces venous return Indication Angina pectoris CHF associated with AMI Cardiac load reducing agent Hypertensive Crisis Side effects CNS: headache, throbbing, dizziness, weakness GI: nausea, vomiting Skin: Rash Adverse Reactions CV: orthostatic hypotension, flushing, fainting. EENT: sublingual burning. Skin: Cutaneous vasodilation, contact dermatitis (patch) Contraindications Hypersensitivity With acute angle closure glaucoma, obstructive uropathy, obstructive disease of GI tract, paralytic ileus, toxic megacolon, intestinal atony, unstable CV status in acute hemorrhage, asthma, or myasthenia gravis. Pregnant women. Contraindications Contraindicated in patients hypersensitive to nitrates With early MI. (S.L. form), severe anemia, increase ICP angle-closure glaucoma, IV nitroglycerine is contraindicated in patients with hypovolemia, hypotension, orthostatic hypotension, cardiac tamponade restrictive cardiomyopathy, constrictive pericarditis. Nursing Management Monitor VS Report é HR Monitor for constipation, oliguria. Instruct to take 30 mins before meals Eat foods high in fiber and drink plenty fluids. Can cause photophobia Instruct client not to drive a motor vehicle or participate in activities requiring alertness. Nursing Management Record characteristics and precipitating factors of anginal pain. Monitor BP and apical pulse before administration and periodically after dose. Have client sit or lie down if taking drug for the first time. Client must have continuing EKG monitoring for IV administration Cardioverter/ defibrillator must not be discharged through paddle electrode overlying Nitro-Bid ointment or the TransdermNitro Patch. Assist with ambulating if dizzy. Instruct to take at first sign of anginal pain. May be repeated q 5 minutes to max. of 3 doses. If the client doesn’t experience relief, advise to seek medical assistance immediately. Keep in a dark colored container MORPHINE SULFATE Immediate-release tablets: MSIR Timed-release: Kadian, M-Eslon (CAN), MS Contin, Oramorph SR Oral solution: MSIR, Rescudose, Roxanol, Roxanol T Rectal suppositories: RMS Injection: Astramorph PF, Duramorph, Epimorph (CAN) Preservative-free concentrate for microinfusion devices for intraspinal use: Infumorph Classification Opioid Agonist Analgesic Dosage Oral: 10–30 mg q 4 hr PO. Controlledrelease: 30 mg q 8–12 hr PO or as directed by physician; Kadian: 20–100 mg PO daily–24-hr release system; MS Contin: 200 mg PO q 12 hr. SC and IM:10 mg (5–20 mg)/70 kg q 4 hr or as directed by physician. IV:2.5–15 mg/70 kg of body weight in 4–5 mL water for injection administered over 4– 5 min, or as directed by physician. Continuous IV infusion: 0.1–1 mg/mL in 5%dextrose in water by controlled infusion device. Rectal:10–30 mg q 4 hr or as directed by physician. Action Acts as agonist at specific opioid receptors in the CNS to produce analgesia, euphoria, sedation Indication Relief of moderate to severe acute and chronic pain Preoperative medication Analgesic adjunct during anesthesia Component of most preparations that are referred to as Brompton's cocktail or mixture Intraspinal use with microinfusion devices for the relief of intractable pain Unlabeled use: Dyspnea associated with acute left ventricular failure and pulmonary edema Side Effects GI: dry mouth, constipation. Skin: Tissue irritation and induration (SC injection). Other: sweating,physical tolerance and dependence, psychological dependence Adverse Effects CNS: Light-headedness, dizziness, sedation, euphoria, dysphoria, delirium, insomnia, agitation, anxiety, fear, hallucinations, disorientation, drowsiness, lethargy, mpaired mental and physical performance, coma, mood changes, weakness, headache, tremor, seizures, miosis, visual disturbances, suppression of cough reflex CV: Facial flushing, peripheral circulatory collapse, tachycardia, bradycardia, arrhythmia, palpitations, chest wall rigidity, hypertension, hypotension, orthostatic hypotension, syncope Dermatologic: Pruritus, urticaria, Respiratory: laryngospasm, bronchospasm, edema GI: Nausea, vomiting, anorexia, biliary tract spasm; increased colonic motility in patients with chronic ulcerative colitis GU: Ureteral spasm, spasm of vesical sphincters, urinary retention or hesitancy, oliguria, antidiuretic effect, reduced libido or potency Respiratory:Respiratory depression, apnea, circulatory depression, respiratory arrest, shock, cardiac arrest Contraindications Hypersensitivity to opioid Diarrhea caused by poisoning until toxins are eliminated During labor or delivery of a premature infant After biliary tract surgery or following surgical anastomosis Pregnancy Labor Nursing Management Caution patient not to chew or crush controlled-release preparations. Dilute and administer slowly Tell patient to lie down during IV administration. Keep opioid antagonist and facilities for assisted or controlled respiration readily available during IV administration. Use caution when injecting SC or IM into chilled areas or in patients with hypotension or in shock Reassure patients that they are unlikely to become addicted Teaching points Take this drug exactly as prescribed. Avoid alcohol, antihistamines, sedatives, tranquilizers, overthe-counter drugs. Swallow controlled-release preparation (MS Contin, Oramorph SR) whole; do not cut, crush, or chew them. Do not take leftover medication for other disorders, and do not let anyone else take your prescription. These side effects may occur: Nausea, loss of appetite, constipation, dizziness, sedation, drowsiness, impaired visual acuity Report severe nausea, vomiting, constipation, shortness of breath or difficulty breathing, rash. VERAPAMIL Calan, Isoptin, Verelan, Covera HS Classification Anti-anginal Anti-arrhythmics Anti-hypertensive Vascular headache suppressants Dosage PO 80-120 mg 3x daily, increases as needed Action Inhibits calcium transport into myocardial smooth muscle cells Decreases SA and AV conduction and prolongs AV node refractory period in conduction tissue Indication Hypertension Angina Pectoris Supraventricular Arrhythmia Atrial flutter/fibrillation Side Effects and Adverse Reactions CNS:abnormal dreams, anxiety, confusion, dizziness and headache EENT: blurred vision, epistaxis and tinnitus CV: arrhythmia, CHF, chest pain, bradycardia, hypotension and palpitations GU: dysuria, nocturia and polyuria GI: abnormal liver function, anorexia, constipation, diarrhea, nausea and vomiting Contraindications Hypersensitivity Sick sinus syndrome 2nd or 3rd degree AV block CHF Cardiogenic shock Concurrent IV beta-blocker Nursing Management Monitor BP and pulse before therapy, during titration and therapy Monitor ECG, I&O, serum potassium and weight. Assess for CHF DILTIAZEM Cardizem, Dilacor, Novo-Diltiazem, Tiamate and Tiazac Classification Anti-anginals Antiarrhythmics Antihypertensive Ca channel blocker Dosage PO: 30-120 mg, 3-4x daily or 60120 mg twice daily as SR capsules IV: 0.25 mg/kg Action Inhibits calcium transport into myocardial smooth muscle cells Systemic and coronary vasodilation Indication Hypertension Angina Pectoris Supraventricular Arrhythmia Atrial flutter/fibrillation Side Effects and Adverse and Reactions CNS:abnormal dreams, anxiety, confusion, dizziness and headache EENT: blurred vision, epistaxis and tinnitus CV: arrhythmia, CHF, chest pain, bradycardia, hypotension and palpitations GU: dysuria, nocturia and polyuria GI: abnormal liver function, anorexia, constipation, diarrhea, nausea and vomiting Contraindications Hypersensitivity Sick sinus syndrome 2nd or 3rd degree AV block CHF Cardiogenic shock Concurrent IV beta-blocker Nursing Management Monitor BP and pulse before therapy, during titration and therapy Monitor I&O and weight Assess for CHF Routine serum digoxin monitoring Arrhythmias with CHF: 200 mg/day Ventricular dysrrhythmias: 150 mg over the 1st 10 mins then slow 360 mg over the next 6 hrs LIDOCAINE Xylocaine Classification CV drugs: Anti-arrhythmics Anesthetic Dosage Arrhythmia: IV: 0.7-1.4 mg/kg body weight. No more 200 mg within 1 hour period IM: 4-5 mg/kg body weight Action than Action Increases electrical stimulation of ventricle and His-purkinje system by direct action on tissues, resulting to decrease depolarization, automaticity and excitability in ventricles during diastolic phase Indication Anesthesia Arrhythmias Control of Status epilepticus Side Effects and Adverse Reactions GI disturbances, bradycardia, hypotension, convulsion, numbness of tongue, muscle twitching, restlessness, nervousness, dizziness, tinnitus, blurred vision, fetal intoxication, light headedness, drowsiness, apprehension, euphoria, vomiting, sensation of heat, respiratory arrest and CV collapse Contraindications Hypersensitivity Heart block Hypovolemia Adams stroke syndromes Infection at site of injection Nursing Management Assess pt before and after therapy Pts infusion must be on cardiac monitor Monitor ECG, if QT or QRS increases by 50% or more, withhold the drug Monitor BP, check for rebound HPN after 1-2 hrs Assess respiratory status, oxygenation and pulse deficits Assess renal and liver function Monitor CNS symptoms Monitor blood levels AMIODARONE Cordarone Classification Anti-arrhythmics Dosage Recurrent ventricular arrhythmias: POà800-1600 mg/day for 1-2 wks PSVT, symptomatic atrial flutter: POà 600-800 mg/day for 1 month Blocks Na channels, prolonging myocardial cell action potential and refractory period Non competitive alpha and beta adrenergic blockage Indication Life threatening recurrent arrhythmias Ventricular fibrillation Ventricular tachycardia Side Effects and Adverse Reactions Exacerbation of arrhythmias, bradycardia, SA node dysfunction, heart block, sinus arrest; flushing, fatigue, malaise, abnormal involuntary movements, ataxia, dizziness, paresthesia, decreased libido, insomnia, headache, sleep disturbances, visual impairment, blindness, corneal microdeposits, photophobia, abnormal taste, nausea, vomiting, constipation, anorexia, abdominal pain, abnormal salivation, coagulation abnormalities, non-specific hepatic disorders, pulmonary inflammation, dyspnea, toxicosis, death, edema, hypo and hyperthyroidism Contraindications Severe sinus node dysfunction 2nd or 3rd degree AV block Hypersensitivity Nursing Management Assess cardiovascular status before therapy Assess pulmonary, hepatic and thyroid function before and during therapy Monitor fluid and electrolytes, I&O, K, Na and Cl Monitor ECG, BP Assess vision PROCAINAMIDE Pronestyl, Procan-SR, Procanbid Classification Antiarrhythmics Dosage Arrhythmias: 50 mg/kg/day in divided doses 3-6 hourly Action Blocks open Na channels and prolongs the cardiac action potential. This results in slowed conduction and ultimately the decreased rate of rise of the action potential may result on the widening of QRS on ECG Indication Supraventricular and ventricular arrhythmias. Treatment of Wolf-ParkinsonWhite Syndrome Side Effects and Adverse Reactions Severe hypotension, ventricular fibrillation and asystole. Drug induced SLE syndrome, blood disorders, fever, myocardial depression, heart failure, agrunulocytosis, psychosis, angioedema, hepatomegaly, skin irritation hypergammaglobulinemia, GI and CNS effects Contraindications Heart block Heart failure Hypotension Myesthenia gravis Digoxin toxicity Lactation Nursing Management Assess cardiovascular status before therapy Assess pulmonary, hepatic and thyroid function before and during therapy Monitor fluid and electrolytes, I&O, K, Na and Cl Monitor ECG, BP Assess vision EPINEPHRINE Injection, OTC nasal solution: Adrenalin Chloride Ophthalmic solution: Epifrin, Glaucon Insect sting emergencies: EpiPen Auto-Injector (delivers 0.3 mg IM adult dose), EpiPen Jr. Auto-Injector (delivers 0.15 mg IM for children) OTC solutions for Nebulization: AsthmaNefrin, microNefrin, Nephron, S2 Classification Beta2 Adrenergic Agonists Dosage Cardiac arrest: 1 mg IV of 1:10,000 solution q 3-5 min; double dose if administering via ET tube Anaphylaxis: 0.1- 1 mg SQ or IM of 1:1000 solution Asthma: 0.1-0.3 mg SQ or IM of 1:10,000 solution Refractory bradycardia and hypotension: 2-10ug/min Action Stimulates beta receptors in lung. Relaxes bronchial smooth muscle. Increases vital capacity Increases BP, é HR, é PR Decreases airway resistance. Indication Asthma Bronchitis Emphysema All cardiac arrest, anaphylaxis Used for symptomatic bradycardia. Relief of bronchospasm occurring during anesthesia Exercised-induced bronchospasm Side Effects: nervousness, tremor, vertigo, pain, widened pulse pressure, hypertension nausea Adverse Effects: headache Contraindications With angle-closure glaucoma, shock (other than anaphylactic shock), organic brain damage, cardiac dilation, arrhythmias, coronary insufficiency, or cerebral arteriosclerosis. Also contraindicated in patient receiving general anesthesia with halogenated hydrocarbons or cyclopropane and in patients in labor (may delay second stage) In conjunction with local anesthesia, epinephrine is contraindicated for use in finger, toes, ears, nose, and genitalia. In pregnant woman, drug is contraindicated. In breast feeding do not use the drug or stop breast feeding. Nursing Management Monitor V/S. and check for cardiac dysrrhythmias Drug increases rigidity and tremor in patients with Parkinson’s disease Epinephrine therapy interferes with tests for urinary catecholamine Avoid IM use of parenteral suspension into buttocks. Gas gangrene may occur Massage site after IM injection to counteract possible vasoconstriction. Observe patient closely for adverse reactions. Notify doctor if adverse reaction develop If blood pressure increases sharply, rapid-acting vasodilators such as nitrates or alpha blockers can be given to counteract VASOPRESSIN Pitressin Classification Pituitary Hormones ADH Dosage Prevent and treat abdominal distention: initially 5 units IM gives subsequent injections q3-4 hours increasing to 10 units if needed. Action Increase permeability of renal tubular epithelium to adenosine monophosphate and water, the epithelium promotes reabsorption of water and concentrated urine Indication Diabetes Insipidus Abdominal Distention GI bleeding Esophageal varices Side Effects and Adverse Reactions CNS: tremor, headache, vertigo CV: vasoconstriction, arrhythmias, cardiac arrest, myocardial ischemia, circumollar pallor, decreased CO, angina GI: abdominal cramps GU:uterine cramps Respi: bronchoconstriction Skin: diaphoresis, gangrene and urticaria Contraindications With chronic nephritis and nitrogen retention Hypersensitivity Nursing Management Give 1-2 glass of H20 to reduce adverse reactions and improve therapeutic response Warm vasopressin in your hands and mixed until it is distributed evenly in the solution Monitor urine Sp. Gravity and I&O to aid evaluation of drug effectiveness MAGNESIUM SO4 Classification Anti-convulsant Anti-arrhythmics Dosage Arrhythmia: IV 1-6 grams over several minutes, then continuous IV infusion 3-20 mg/min for 548 hours. CV: hypotension, flushing, bradycardia, circulatory collapse, depressed cardiac function EENT: diplopia Respiratory: respiratory paralysis Metabolic: hypocalcemia Skin: diaphoresis Contraindications Heart block and myocardial damage Toxemia of pregnancy Nursing Management Monitor I&O. make sure urine output is 100 ml or more in 4 hrs pd before each dose Take appropriate seizure precautions Keep IV Ca gluconate at bedside Na HCO3 Arm and Hammer; Baking Soda Classification Alkalinizers Dosage Metabolic Acidosis: Usually 2-5 meq/kg IV infuse over 4-8 hr period Cardiac Arrest: 1 meq/kg IV of 7.5 or 8.4% sol, then 0.5 meq/kg IV q 10 mins depending on ABG Action Restore buffering capacity of the body and neutralizes excessive acid Indication Metabolic Acidosis Cardiac Arrest Side Effects/Adverse Reactions CNS: tetany CV: edema GI: gastric distention, belching and flatulence Metabolic: hypokalemia, metabolic alkalosis, hypernatremia, hyperosmolarity with overdose Skin: pain @ injection site Action Decreased acetylcholine released Contraindications Pt losing Cl because of vomiting or continuous GI suction or those receiving diuretics that produces hypochloremic alkalosis Metabolic and respiratory alkalosis Indication Mg replacement Arrhythmia Nursing Management Obtain blood pH, PaO2, PaCo2 and electrolyte levels Side Effects and Adverse Reactions CNS: drowsiness, depressed reflexes, flaccid paralysis, hypothermia HYPERTENSIVE CRISIS Na NITROPRUSSIDE Nittropress Classification Antihypertensive, Vasodilator Dosage 0.25-0.3 mcg/kg/minute Action Relaxes arteriolar and venous smooth muscle Indication Hypertensive crisis To produce controlled hypotension during anesthesia To reduce preload and afterload in cardiogenic shock Side Effects/Adverse Reactions Headache, dizziness, increased ICP, loss of consciousness, restlessness, bradycardia, nausea, abdominal pain, methemoglodinemia, muscle twitching, pink-colored rash, irritation at infusion site Contraindications Hypersensitivity Anuria Nursing Management Monitor wt., BP and PR Monitor fluid, I&O, electrolyte, BUN and CO2 levels frequently WOF signs of hypokalemia Monitor uric acid levels Monitor glucose levels esp in DM pts MORPHINE SO4 (Discussed earlier) NEUROSURGICAL DRUGS MANNITOL Osmitrol Classification Diuretics Dosage Contraindications Hypersensitivity Compensatory hypotension Inadequate cerebral circulation Acute heart failure with reduced PVR Congenital optic atrophy Tobacco-induced ambylopia Nursing Management Obtain VS before giving the drug Place pt in supine Giving excessive doses of 500 mcg/kg delivered faster than 2 mcg/kg/min or using max infusion rate of 10 mcg/kg/min for more than 10 mins can cause cyanide toxicity FUROSEMIDE Lasix Classification Loop Diuretics Dosage Pulmonary edema: 40 mg IV Edema: 20 to 80 mg PO every day in the morning HPN: 40 mg PO bid. Dosage adjusted based on response Action Inhibits Na and Cl reabsorption at the proximal and distal tubules and in the ascending loop of Henle Indication Acute pulmonary edema Edema Hypertension Side Effects/Adverse Reactions Signs of hypotension, hypokalemia and hyperglycemia Test dose for marked oliguria or suspected inadequate renal function: 200 mg/kg or 12.5 gram as a 15% to 20% IV solution over 3-5 mins response is adequate if 3050 ml of urine/hr is adequate, a second dose is given if still no response after 2nd dose stop the drug Oliguria: 50 over 90 mins to several hrs To induced intraocular or intracranial pressure: 1.5-2 gram/kg as a 15 % to 20% IV solution over 30-60 min Diuresis in drug intoxication: 12.5% to 10% solutions up to 200 g IV Irrigating solution during TURP: 2.5-5% Action Increases osmotic pressure of glomerular filtrate, inhibiting tubular reabsorption of water and electrolytes; drug elevates plasma osmolarity, increasing water flow into extracellular fluid Indication Test dose for marked oliguria or suspected inadequate renal function Oliguria To induced intraocular or intracranial pressure Diuresis in drug intoxication Irrigating solution during TURP Side Effects/Adverse Reactions CN: seizures, headache and fever CV: edema, thrombophlebitis, hypotension and heart failure EENT: blurred vision and rhinitis GI: thirst, dry mouth, nausea, vomiting and diarrhea GI: urine retention Metabolic: dehydration Skin: local pain Others: chill Respiratory rate increases within 1-2 mins IPECAC SYRUP Contraindications Hypersensitivity Anuria, severe pulmonary congestion, frank pulmonary edema, active intracranial bleeding during craniotomy, severe dehydration, metabolic edema, progressive heart failure or pulmonary congestion after drug Classification Antidote Nursing Management Monitor VS,CVP,I&O, renal function fluid balance and urine K levels daily. Drug can be used to measure GFR Do not give electrolyte free solutions with blood. If blood id given simultaneously, add at least 200 meq of NaCL to each liter Indication Poisoning Overdose POISONING NALOXONE HCL Narcan Classification Miscellaneous antagonists and antidotes Dosage For suspected opioid induced respiratory depression: 0.4 to 2 mg IV, IM and SQ. repeat doses q 2-3 mins PRN For postoperative opiod depression: 0.01 to 0.2 mg IV q 2-3 mins, PRN. Repeat dose within 1-2 hr, if needed. Action Reverse the effects of opiods, psychotomimetic and dysphoric effects of agonist-antagonists Indication For suspected opioid induced respiratory depression For postoperative opiod depression Side Effects/Adverse Reactions CNS: seizures, tremors CV: ventricular fibrillation, tachycardia, HPN with higher recommended doses, Hpotension GI: nausea and vomiting Respiratory: pulmonary edema Skin: diaphoresis Contraindications Hypersensitivity Use cautious with cardiac irritability or opiod addiction. Nursing Management Assess respiratory status frequently Dosage 25-30 ml followed immediately by H2O Action Irritates the stomach lining and stimulate the vomiting center Side Effects Diarrhea, drowsiness, stomach cramps, vomiting, itching, DOB, swelling of the mouth, rash and hives Contraindications Hypersensitivity Given activated charcoal Unconcious Drowsy Severely drunk Having seizures With no gag reflex Nursing Management Don’t administer to unconscious Pt should kept active and moving ff administration If vomiting does not occur after 2nd dose, gastric lavage may be considered to remove ingested substance ACTIVATED CHARCOAL Classification Antidote Dosage 30-100 g with at least 8 oz of water Action Inhibits GI absorption of toxic substances or irritants Hyperosmolarity Indication Poisoning Side Effects Pain, melena, diarrhea, vomiting and constipation Contraindications Cyanide, mineral acids, organic solvents, intestinal obstruction, bleeding with fructose intolerance, broken GI tract, concomitant use of charcoal with sorbitol Nursing Management Do not mix with chocolate and together with ipecac syrup Notify doctor if caused swelling or pain in the stomach FLUMAZENIL Romazicon Classification Benzodiazepine receptor antagonists Dosage 2 ml IV given over 15 seconds Action Antagonizes the effects of benzodiazepines Indication Benzodiazepine-induced depression of the ventilatory responses to hypercapnia and hypoxia Side Effects Nausea, vomiting, palpitations, sweating, flushing, dry mouth, tremors, insomnia, dyspnea, hyperventilation, blurred vision, headache, pain at injection site Contraindications Control of ICP or status epilepticus. Signs of serious cyclic antidepressant overdose Nursing Management Must individualize dosage. Give only smallest amount effective. Give through freely running IV infusion into large vein to minimize pain at injection site Note history of seizure or panic disorder Assess evidence of increased ICP Note evidence of sedative and benzodiazepine dependence Instruct to avoid alcohol and nonprescription drugs for 1-24 hrs SHOCK DOPAMINE Intropine Classification Adrenergic drugs Dosage Initially 2-5 mcg/kg/min by IV Action Stimulates dopaminergic and alpha and beta receptors of the sympathetic nervous system resulting in positive inotropic effect and increased CO Indication To treat shock and correct hemodynamic imbalance To correct hypotension To improve perfusion of vital organs To increase CO Side Effects CNS: headache and anxiety CV: tachy, angina, palpitations and vasoconstriction GI: nausea and vomiting Contraindications Hypersensitivity With uncorrect tachyarrhythmias Pheochromocytoma Ventricular Fibrillation Nursing Management Most patients received less than 20 mcg/kg/min Drugs isn’t substitute for blood or fluid volume deficit During infusion, monitor ECG, BP, CO, PR and color and temp of the limbs Do not confuse dopamine to dobutamine Check urine output often DOBUTAMINE Dobutrex Classification Adrenergic drugs Dosage 0.5-1 mcg/kg/min IV infusion, titrating to optimum dosage of 2-20 mcg/kg/min 2.5 to 10 mcg/kg/min-usual effective range to increase CO Action Stimulates heart beta receptors to increase myocardial contractility and SV Indication To increase CO Treatment of cardiac decompensation Side Effects CNS: headache CV: HPN, tachycardia, palpitations and vasoconstriction GI: nausea and vomiting Contraindications Hypersensitivity Use cautiously in pts with hx of HPN and AMI Nursing Management Before starting therapy, give a plasma volume expander to correct hypovolemia and a cardiac glycoside Monitor ECG, BP, pulmonary artery wedge pressure and CO Monitor electrolyte levels Don’t confuse dobutamine to dopamine DIPHENHYDRAMINE HCL Benadryl Classification Anti-histamine Dosage 25-50 mg PO, IV or IM bid-tid GLUCAGON Classification Pancreatic Hormones Dosage 0.5-1 mg SQ, IV, IM, repeat in 20 mins PRN Action Binds with glucagon receptor Indication Hypoglycemia Side Effects Nausea, vomiting, hypotension, tachycardia and hypertension Contraindications Hypersensitivity Pheochromocytoma Insulinoma Nursing Management Monitor V/S and blood sugar level Response within 20 mins after injection ALBUTEROL Ventolin Classification Bronchodilator, Adrenergic Dosage 2 inhalations reputed q 4-6 hrs via neb Action Activation of beta adrenergic receptors on airway smooth muscle Indication Asthma Prevention of exercise induced spasms Side effects Palpitations Tachycardia GI upset Nervousness Contraindications Hypersensitivity Nursing Management Monitor therapeutic effectiveness Monitor HR, BP, ABG, s/sx of bronchospasm and CNS stimulation Instruct on how to use inhaler properly Rinse mouth after use Action Blocks the effects Hi receptor sites Indication Allergic reactions Motion sickness Cough suppression Sedation Side Effects Xerostomia Urinary retention Sedation Contraindications Acute asthmatic attack Nursing Management Risk for photosensitivity- use sunscreen EPINEPHRINE (Discussed earlier)